Fig. 2. Delivery to skin using microneedles.
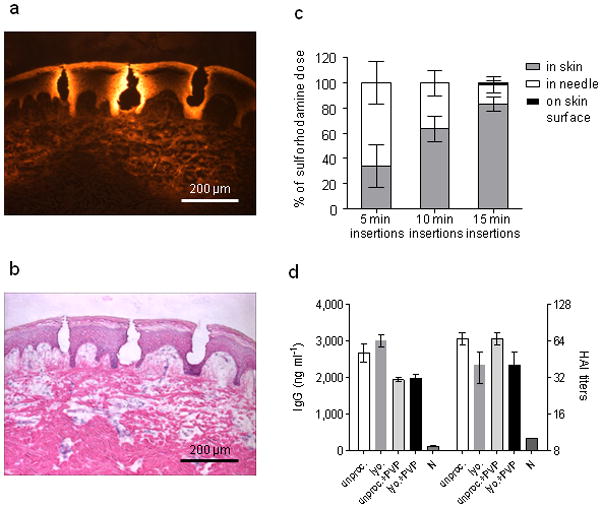
(a) Fluorescence micrograph of pig skin histological section after insertion of dissolving microneedles in vitro. (b) Brightfield micrograph of the same image with H&E staining. (c) Dissolving microneedle delivery efficiency to mice in vivo. Sulforhodamine was encapsulated within microneedles and administered to mice. The delivery efficiency was determined by measuring the amount of sulforhodamine left in microneedles after insertion as well as on the skin surface of the mouse. The remaining sulforhodamine was considered to be delivered to the skin. n = 5 for each time point. The delivery efficiencies for the three time points were statistically different from one another (Student’s t-test, P < 0.05). (d) mice (n = 3) were immunized IM with 20 μg inactivated influenza virus (A/PR/8/34) after different processing and formulation. Serum IgG antibody titers and HAI were measured 14 days after immunization. Antigen lyophilization, mixture with PVP and encapsulation in microneedles had no effect on IgG or HAI titers. Groups: unproc.: unprocessed inactivated influenza virus in PBS; lyo: lyophilized, re-dissolved in PBS inactivated influenza virus; encaps. + PVP: lyophilized inactivated influenza virus encapsulated in PVP; unproc. + PVP: unprocessed inactivated influenza virus in PBS mixed with PVP; N: naïve mice.
