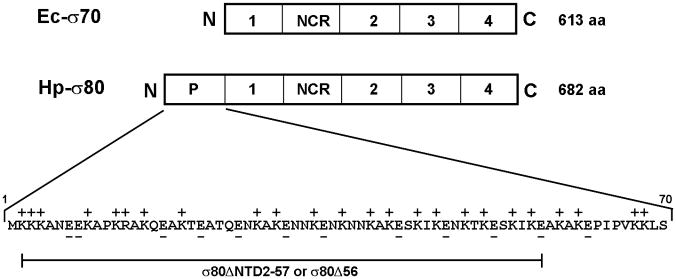
Figure 3. PolyP binds to the Lys-rich region at the NTD of σ80.
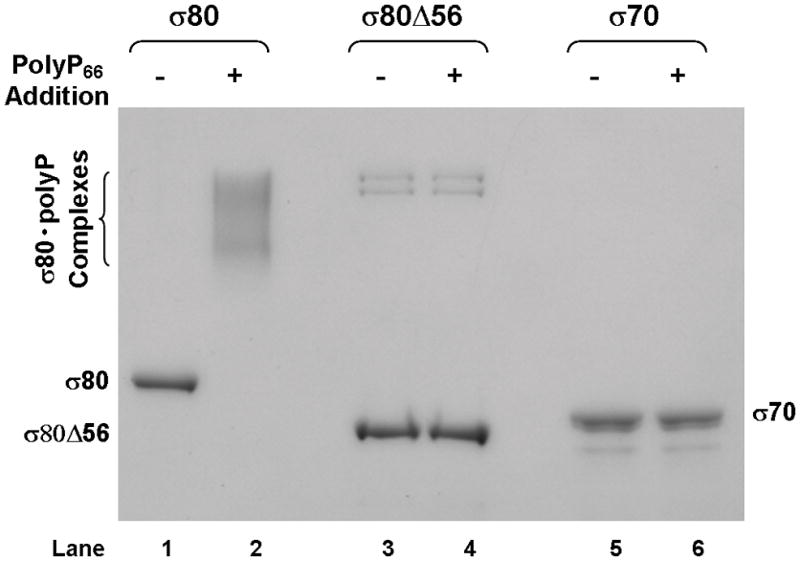
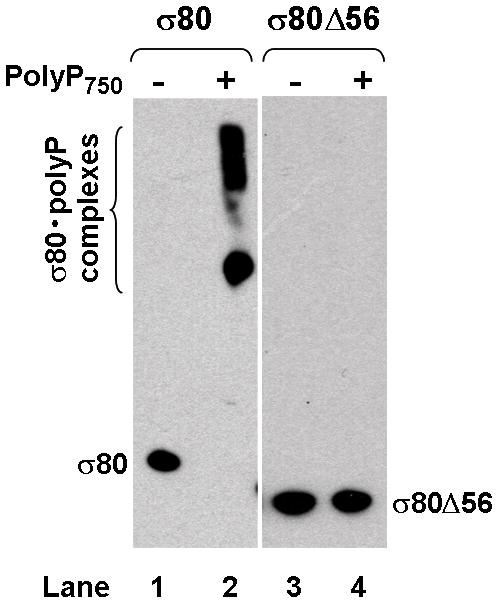
(A) There is an extra region of about 70 amino acid residues (aa) at the NTD of the H. pylori [NC_011333] σ80 compared with the E. coli [U00096] σ70. This extra region is concentrated with positively charged (+) aa including 23 Lys and one Arg, compared with 12 negatively charged (−) Glu. This extra region is named “region P” because it is a positively-charged-Lys-rich region and the target for polyP (see text). The conserved regions and non-conserved region (NCR) of members of the σ70 family proteins (Gruber & Gross, 2003) are shown. The rpoD mutation that deleted the aa 2–57 (σ80ΔNTD2-57 or σ80Δ56) is indicated. (B) A putative “region P” of the primary σ factor from Bordetella pertussis [NC_002929] or Coxiella burnetii (NC_002971). For simplicity, only the positively-charged-Lys-rich extra region (aa before conserved “region 1”) at the NTD of the σ factor indicated is shown. Positively charged (+) and negatively charged (−) aa are indicated. (C) Region P of σ80 is important for binding polyP. Purified recombinant σ80, σ80Δ56 and σ70 proteins in the absence (−) or presence (+) of polyP66 were analyzed by 4–12% SDS-PAGE. The gels were stained with QuickBlue Stain. The positions of σ80, σ80Δ56, σ70 and the σ80·polyP complexes are indicated. The other minor bands reflected impurities in protein preps. In particular, the higher molecular weight bands in lanes 3 & 4, were not related to σ80Δ56 as they were not immunostained by the σ80–specific polyclonal antibodies (data not shown). (D) PolyP is coimmunoprecipitated with the wild type σ80 protein but not with the NTD deletion under native non-denaturing condition. Purified recombinant σ80 and σ80Δ56 proteins in the absence (−) or presence (+) of polyP750 were immunoprecipitated by anti-polyHistidine antibody, and the samples were analyzed as described in the legend of Figure 1. The positions of σ80, σ80Δ56 and the σ80·polyP complexes are indicated.