Abstract
Rationale
Notch signaling regulates vascular development. However, the implication of the Notch ligand Delta-like 4 (Dll4) in postischemic angiogenesis remains unclear.
Objective
We investigated the role of Dll4/Notch signaling in reparative angiogenesis using a mouse model of ischemia.
Methods and Results
We found Dll4 weakly expressed in microvascular endothelial cells of normoperfused muscles. Conversely, Dll4 is upregulated following ischemia and localized at the forefront of sprouting capillaries. We analyzed the effect of inhibiting endogenous Dll4 by intramuscular injection of an adenovirus encoding the soluble form of Dll4 extracellular domain (Ad-sDll4). Dll4 inhibition caused the formation of a disorganized, low-perfused capillary network in ischemic muscles. This structural abnormality was associated to delayed blood flow recovery and muscle hypoxia and degeneration. Analysis of microvasculature at early stages of repair revealed that Dll4 inhibition enhances capillary sprouting in a chaotic fashion and causes excessive leukocyte infiltration of ischemic muscles. Furthermore, Dll4 inhibition potentiated the elevation of the leukocyte chemoattractant CXCL1 (chemokine [C-X-C motif] ligand 1) following ischemia, without altering peripheral blood levels of stromal cell–derived factor-1 and monocyte chemoattractant protein-1. In cultured human monocytes, Dll4 induces the transcription of Notch target gene Hes-1 and inhibits the basal and tumor necrosis factor-α-stimulated production of interleukin-8, the human functional homolog of murine CXCL1. The inhibitory effect of Dll4 on interleukin-8 was abolished by DAPT, a Notch inhibitor, or by coculturing activated human monocytes with Ad-sDll4–infected endothelial cells.
Conclusions
Dll4/Notch interaction is essential for proper reparative angiogenesis. Moreover, Dll4/Notch signaling regulates sprouting angiogenesis and coordinates the interaction between inflammation and angiogenesis under ischemic conditions.
Keywords: Dll4, Notch signaling, angiogenesis, inflammation, ischemia
The Notch signaling pathway is implicated in cell–cell contact and communication, which are both important for the control of cell growth, differentiation, and specification.1 Functional Notch signaling is definitely required for normal vascular development, being part of the genetic program that determines the arterial and venous endothelium identity.2-4 In addition, growing evidence supports the in-volvement of the Notch ligand Delta-like (Dll)4 in the regulation of sprouting angiogenesis in the murine retina and developing zebrafish.5,6 The balance between sprouting and tube formation is essential for the generation of a new functional vessel. This process is reportedly modulated by Dll4 signals from tip endothelial cells (ECs) to neighboring stalk ECs to restrict the emergence of excessive sprouting through repression of VEGFR2 (vascular endothelial growth factor receptor 2) transcription and consequent reduction of responsiveness to vascular endothelial growth factor.7,8 Blockade of Dll4-mediated signaling reportedly slows down tumor growth despite an increase of tumor vasculature density because the established vascular network is functionally inefficient.9-11 However, the implication of Dll4 in reparative neovascularization after ischemia remains unexplored.
The soluble form of Dll4 extracellular domain (sDll4) has been used to inhibit Dll4/Notch interaction in vitro and in vivo.11-14 In line with this, we used an inhibitory approach based on the local gene delivery of sDll4 to investigate the importance of endogenous Dll4 signaling in a mouse model of peripheral ischemia. Results show that Dll4 is upregulated in ischemic tissues. Importantly, inhibition of Dll4/Notch signaling by sDll4 resulted in deleterious consequences for the morphogenesis and function of postischemic neovascularization, featuring a chaotic capillary sprouting and massive leukocyte infiltration.
Methods
An expanded Methods section is available in the Online Data Supplement at http://circres.ahajournals.org.
Animal Model
All experimental procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals (NIH, 1996) and with approval of UK Home Office. Eight-week-old male CD1 mice (Harlan) underwent unilateral femoral artery ligation as described.15 At the same occasion, an adenovirus vector carrying sDll4 (Ad-sDll4) or control vectors (either Ad-Null or Ad-β-galactosidase [Ad-β-Gal]) was injected at 2 sites of the adductor muscle and at 1 site into gracilis and digitalis flexor muscles each (total Ad dose: 2×108 plaque-forming units in 40 μL). Blood flow recovery to the ischemic foot was sequentially monitored by color laser Doppler up to 14 days after ischemia.16 Mice were euthanized in groups at 3, 5, 7, or 14 days.
Real-Time Polymerase Chain Reaction
Total RNA was extracted from isolated adductor muscles at 3, 5, and 14 days after ischemia using TRIzol (Invitrogen). Real-time polymerase chain reaction (PCR) was carried out using the QuantiTect SYBR Green PCR Kit (Qiagen). To detect transgenic sDll4 in muscles, we designed specific primers that recognize only the extracellular domain of human Dll4 (5′-TCCAACTGCCCTTCAATTTCAC-3′; 5′-CTGGATGGCGATCTTGCTGA-3′). Validated primers were purchased (Qiagen). The relative expression ratio was calculated from the real-time PCR efficiencies and crossing point deviation of treated samples versus controls. Values were then normalized to house-keeping gene 18S rRNA or β-actin in each sample according to Pfaffl method.17
Immunoblotting
Western blotting was performed using antibodies against human Dll4 (1:500) and α-tubulin (1:1000) (Abcam) followed by appropriate horseradish peroxidase–conjugated secondary antibodies.
Whole-Mount Immunohistochemistry
At 5 or 14 days postischemia, anesthetized mice were perfused/fixed under physiological pressure with 4% paraformaldehyde. Adductor muscles or hearts were isolated, carefully dissected under stereomi–croscope, and snap-frozen. Samples were cut into slices of 100-μm thickness (Online Figure I). Briefly, following incubation with blocking buffer, the samples were incubated with primary antibody overnight at 4°C. Further incubation with appropriate secondary antibody was performed. Serial z-stack images of adductor muscles were generated using confocal microscopy.
Assessment of Muscular Hypoxia
Hypoxyprobe-1 (pimonidazole hydrochloride, Chemicon) (60 mg/ kg) was injected intraperitoneally 90 minutes before animal termination (7 days postischemia). Isolated adductor muscles were homogenized and used for hypoxyprobe-1 adducts measurement by ELISA. The values obtained from the ischemic side were normalized to the nonischemic contralateral side.18
Flow Cytometric Analysis
Isolated adductor muscles were digested with 0.2% collagenase type II/DMEM for 30 minutes at 37°C. The digest was filtered by 40-μm mesh to obtain a single cell suspension. Cells were then stained with anti–CD45-APC antibody (1:100, BD Biosciences). Analysis was performed after gating against propidium iodide, which allowed us to identify and exclude necrotic cells. Analysis was performed using a FACS Canto II equipped with a FACS Diva software (BD Biosciences).
Cell Culture
The inhibitory activity of sDll4 on the Dll4/Notch signaling was verified by measuring the expression of Notch target gene, Hes1, which is transcriptionally upregulated by Notch in human umbilical vein ECs (HUVECs) activated with immobilized Dll4. Differentiated THP1 human monocyte cell line was cocultured with HUVECs transfected with Ad-sDll4 or Ad-Null to examine the effect of Dll4/Notch signaling on interleukin (IL)-8 secretion from monocytes.
Matrigel Assay
HUVECs were transfected with Ad-sDll4 or Ad-Null and cocultured with DiI-labeled THP1 monocytes on Matrigel. The dynamic interaction between the 2 cell types during the process of endothelial network formation was monitored by time lapse phase-contrast video microscopy.
Enzyme-Linked Immunosorbent Assays
Measurement of human Dll4 in peripheral blood of mice injected with Ad-sDll4 or Ad-Null was performed by direct ELISA using antibody antihuman Dll4 (Abcam). Plasma levels of monocyte chemotactic protein-1, stromal cell–derived factor-1, and chemokine (C-X-C motif) ligand 1 (CXCL1) (murine functional homolog of human IL-8) were measured by ELISA kits (R&D Systems). An ELISA kit (R&D Systems) was used to measure the levels of IL-8 in the conditioned media from human monocytes.
Statistics
Comparison of multiple groups was performed by ANOVA. Two-group analysis was performed by Student's t test. Values were expressed as means±SEM. Probability values of less than 0.05 were considered significant.
Results
Dll4 Is Expressed in Newly Forming Vessels After Ischemia
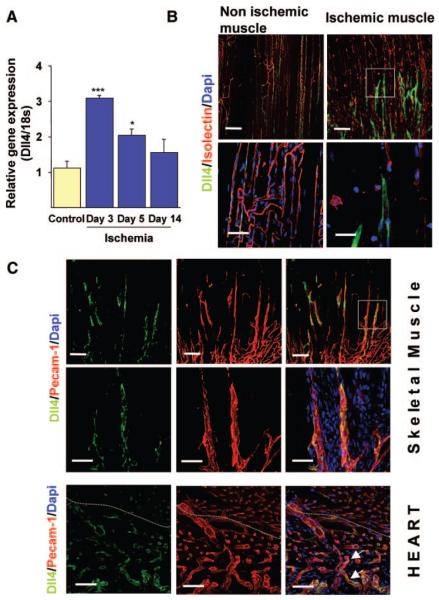
Previous studies reported Dll4 to be expressed in embryonic and tumor vasculature.19,20 We first determined whether Dll4 is expressed in adult skeletal muscles under normal conditions and after ischemia. Dll4 transcription is induced in adductor muscles after femoral artery ligation, with peak of mRNA expression at 3 days (Figure 1A). Whole-mount immunohistochemical analysis using specific anti-Dll4 antibodies revealed weak and patchy expression of Dll4 in microvascular ECs of normoperfused muscles. Conversely, Dll4 expression was upregulated in capillary ECs after ischemia (Figure 1B). We found Dll4 mainly expressed in capillary sprouts, which coexpressed platelet endothelial cell adhesion molecule (PECAM)-1 but were negative to GS IsolectinB4 (Figure 1B and 1C). Failure of Dll4-positive neovessels to express Isolectin-binding sites confirms their immaturity. Of note, the expression of Dll4 is more pronounced in the angiogenic front in the early phase of regeneration (Figure 1C). Similarly, Dll4 was detected in infarcted hearts at the level of neovessels within the periinfarct region (Figure 1C).
Figure 1. Upregulation of Dll4 in ischemic tissues.
A, Time course of Dll4 transcription in skeletal muscles measured by quantitative PCR. *P<0.05 vs control (n=6 each time point). B, Confocal images of adductor muscles showing the induction of Dll4 expression in newly forming capillaries, 5 days postischemia. Dll4 (green), IsolectinB4 (marker of ECs, red), DAPI (nucleus marker, blue). Scale bar: 100 μm. Bottom images, Highermagnification images showing the high expression of Dll4 in new capillary sprouts. Scale bar: 50 μm. C, Confocal images showing costaining of Dll4 (green) and PECAM-1 (EC marker, red). Scale bar: 100 μm. Bottom images, High magnification of upper panels. Scale bar: 50 μm. D, Dll4 expression in the myocardium 5 days after myocardial infarction. Scale bar: 50 μm. The dotted line delimits the infarct border zone. Arrows point ECs double positive for Dll4 and PECAM-1.
Inhibition of Dll4/Notch Signaling by Ad-sDll4
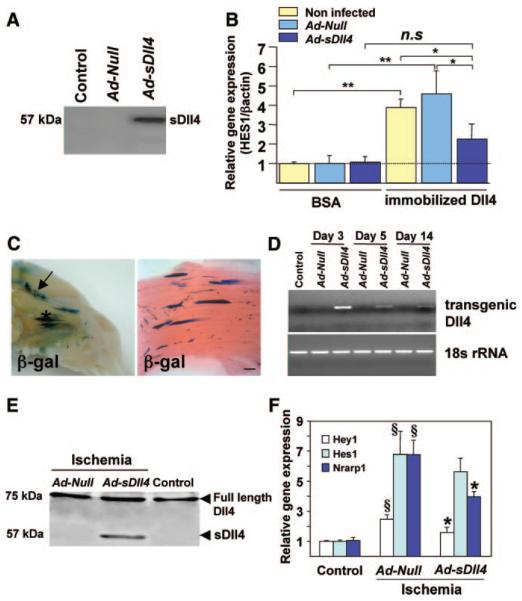
To assess the efficiency of transgenic sDll4 to block endogenous Dll4, we measured mRNA expression of Hes-1 (Notch downstream gene) in HUVECs activated with immobilized Dll4 or seeded on BSA (control of immobilized Dll4) and cocultured with Null- or sDll4-tranduced HUVECs or with noninfected HUVECs. Production of transgenic sDll4 in the conditioned culture medium was assessed by immunoblotting (Figure 2A). As expected, Hes-1 was significantly upregulated by immobilized Dll4 in the presence of either noninfected HUVECs or Null- HUVECs (P<0.01 versus BSA for both comparisons) but repressed in the presence of sDll4- HUVECs (P<0.05 versus either Ad-Null or noninfected, Figure 2B).
Figure 2. Inhibition of Dll4-mediated signaling by Ad-sDll4.
A, Western blotting showing the soluble protein of Dll4 in supernatant of Ad-sDll4–infected HUVECs. B, Real-time PCR results showing the induction of Hes-1 mRNA levels in HUVECs seeded on immobilized Dll4 and the inhibition of Hes-1 upregulation by coculture with HUVECs transfected with Ad-sDll4. Values are fold changes relative to Hes-1/β-actin ratio in control (BSA, noninfected); n 3 replicates. *P>0.05, **P>0.01. C, Representative photograph of exposed hindlimb muscles showing distribution of β-galactosidase (β-gal) 5 days after Ad-β-gal injection (arrow shows site of femoral artery ligation). Right, Longitudinal section of adductor muscle showing β-gal staining counterstained with eosin. Scale bar: 200 μm. D, Time course of transgenic Dll4 mRNA levels in whole homogenized adductor muscles following Ad-sDll4 injection. E, Immunoblot represents the presence of extracellular domain of Dll4 (sDll4) (57 KDa only in Ad-sDll4–injected muscles). F, Induction of Notch target genes in adductor muscles at 3 days postischemia and inhibition of Hey-1 and Nrarp-1 upregulation by Ad-sDll4; §P<0.001 vs contralateral muscles; *P<0.05 vs Ad-Null; n=6 each group.
For in vivo experiments, the ability of the adenoviral vector to infect mouse hindlimb muscles and its tissue distribution was preliminarily conformed following Ad-β-Gal delivery. We found muscle fibers infected with Ad-β-Gal vector as identified by X-Gal staining (Figure 2C). Next, we examined the efficiency of Ad-sDll4 infection in ischemic muscles. We found that expression of transgenic sDll4 in adductor muscles peaked at day 3 after gene transfer and declined thereafter, remaining detectable until at least 14 days (Figure 2D). Hence, the time course of transgenic sDll4 was well synchronized with the uprising of endogenous Dll4. Furthermore, sDll4 protein was detected in Ad-sDll4–injected muscles (Figure 2E) but not in peripheral blood (Online Figure II). The ability of Ad-sDll4 to block Dll4/Notch signaling in vivo was tested by measuring mRNA expression of Notch target genes: Hes-1, Hey-1, and Nrarp-1 (Notch-regulated ankyrin repeat protein). As shown in Figure 2F, in Ad-sDll4–injected muscles, Hey-1 and Nrarp-1 mRNA levels were reduced in comparison to Null muscles.
Inhibition of Dll4/Notch Signaling Impairs Reperfusion and Healing of Ischemic Muscles
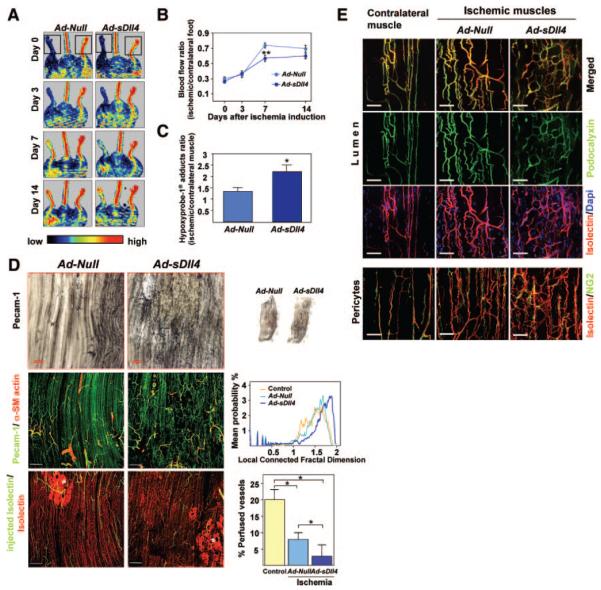
Postischemic foot blood flow recovery was sequentially measured by color laser Doppler. At 7 days postischemia induction, 73±3% of foot blood flow was restored in Ad-Null-mice, whereas recovery was only 56±4% in Ad-sDll4 mice (P=0.002 versus Ad-Null; n=11 mice per group) (Figure 3A and 3B). Moreover, the levels of hypoxyprobe-1 adducts (hypoxia indicator) were significantly increased in muscles injected with Ad-sDll4 (2.2±0.3 versus1.3±0.2 in Ad-Null at day 7, P=0.03, n=5 mice per group) (Figure 3C).
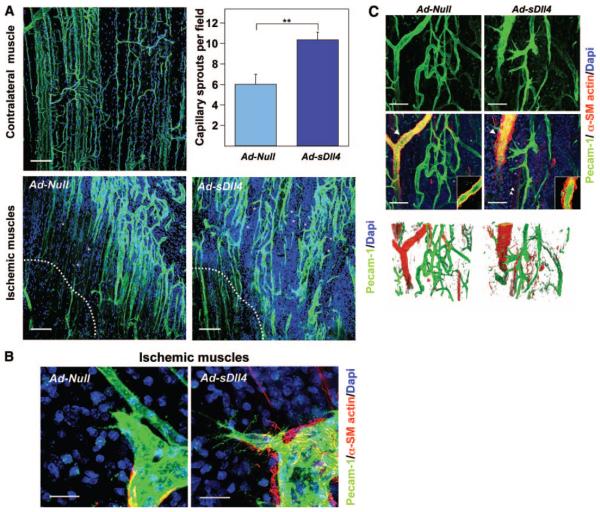
Figure 3. Dll4 inhibition impairs reperfusion and neovascularization of ischemic muscles.
A, Representative color-coded images of laser Doppler flowmetry. The dotted square indicates the feet, where blood flow was measured. B, Line graph showing the time course of blood flow. *P=0.002 vs Ad-Null; n=11 mice per group. C, Levels of hypoxyprobe-1 adducts (ischemic to contralateral muscle ratio) as an index of residual muscular hypoxia. *P=0.03 vs Ad-Null; n=5 per group. D, Top right images, Micrographs, taken at 14 days postischemia, showing whole ischemic adductor muscles stained with PECAM-1 (black). Top left images, Higher magnifications showing high density of vascular network in Ad-sDll4–injected muscles compared to Ad-Null. Middle left images, Representative confocal microscopy photographs taken at 14 days postischemia. ECs are stained with PECAM-1 (green), and vascular smooth muscle cells are stained with α-smooth muscle actin (red). Middle right images, Line graph illustrates local-connected fractal dimension analysis. Bottom left images, Representative confocal images showing perfused microvessels stained in vivo by biotinylated lectin (green). Samples were counterstained with lectin-Alexa 568 to label all vascular structure (red: total vessels). Asterisks show necrotic areas catch up unspecifically Isolectin. Scale bar: 200 μm. Bottom right graphs, Bar graphs showing average perfused vessel ratio. *P>0.05; n=10 mice per group. E, Representative confocal microscopy photographs taken at 14 days postischemia show similar lumen formation (podocalyxin, green) and vascular pericyte coverage (NG2, green) in ischemic limb muscles of the 2 groups. Contralateral normoperfused muscle is shown as reference. Scale bar: 50 μm.
Next, we examined the effect of Dll4/Notch signaling inhibition on reparative neovascularization. Whole-mount immunohistochemical examination of adductor muscle microvasculature at 14 days postischemia demonstrated the regular alignment of newly forming capillaries in muscles injected with Ad-Null, whereas Ad-sDll4–injected muscles featured an irregularly shaped capillary network (Figure 3D). Total vascular area and capillary density were both increased in Ad-sDll4–injected ischemic muscles compared to Ad-Null (Online Figure III, A and B). Given the peculiar architecture of capillary network, fractal dimension (Df) was used to describe the complexity of microvasculature as visualized by whole-mount staining.21 We found a higher level of complexity in Ad-Null-injected ischemic muscles compared to normoperfused muscles (1.676±0.017 versus 1.614±0.008, respectively; P=0.01, n=5) and a further increase in complexity in Ad-sDll4–injected muscles (1.743±0.007 versus Ad-Null, P=0.008, n=5). Furthermore, analysis of local-connected fractal dimension (LCFD) was performed to measure the local variation in density of the whole vascular network. Distribution of LCFD shows that the microvasculature of Ad-sDll4–injected muscles has a higher probability of high-dimensional features (1.72 to 2 region), which corresponds to high dense and branched areas, and a lower probability of low-dimensional features (1.2 to 1.6 region), which refers to the normal distribution for microvasculature of normoperfused muscles (Figure 3D).
Despite of the apparent increase of vascular network density, the number of perfused vessels stained by intracardially injected Isolectin was remarkably decreased in Ad-sDll4–injected muscles (Figure 3D). Furthermore, perfused vessels showed smaller caliber compared to Ad-Null. The maturation of neovessel requires recruitment of mural cells and formation of lumen for structural support and regulation of tissue perfusion. Here, the examination of capillaries revealed that pericyte coverage or lumen formation was not affected by Ad-sDll4. Similarly, the abundance of vascular smooth muscle cells (VSMCs) in arterioles showed no significant difference between groups (Figure 3E).
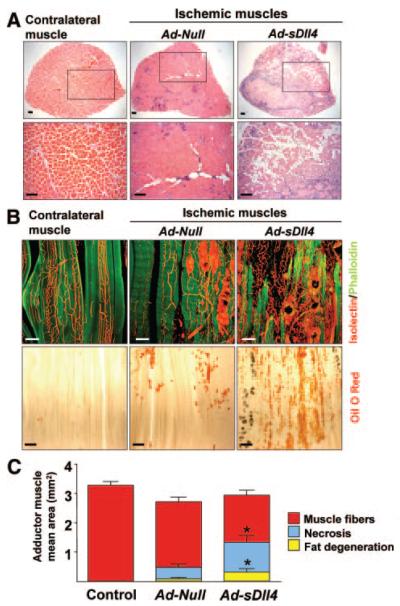
Proper adaptive angiogenesis is critical for muscle regeneration and healing after ischemia. Importantly, Ad-sDll4 produced a marked loss of skeletal myocytes (Figure 4A and 4C), which were substituted by fat accumulation (Figure 4B and 4C). Taken together, these findings suggest that Dll4 mediated signaling inhibition results in the formation of an inefficient neovessel network unable to support postischemic muscle recovery.
Figure 4. Dll4 Inhibition impairs muscle regeneration.
A, Cross-section showing the whole perimeter of adductor muscles harvested at 14 days postischemia and stained with hematoxylin/eosin. Lower panel: higher magnification of upper panel. B) Considerable loss of muscle fibers in Ad-sDll4–injected ischemic muscles. Myocytes (phalloidin, green), microvessels (IsolectinB4, red). Scale bar: 100 μm. Bottom images, Lipid deposit is increased in Ad-sDll4–injected ischemic muscles as revealed by oil red O staining (red). Scale bar: 200 μm. C, Quantification of muscle fibers, lipid degeneration, and necrosis area. *P<0.05; n=6 per group.
Inhibition of Dll4/Notch Signaling Causes Chaotic Sprouting Angiogenesis
To elucidate the cellular mechanisms underlying of the observed defects, we analyzed the morphology of neovascularization on earlier stage of reparative angiogenesis at day 5 postischemia. Forced expression of sDll4 resulted in a 40% increase of capillary sprouts (P=0.003 versus Ad-Null, n=10 per group). The capillary sprouts were directed toward necrotic region in Ad-Null injected ischemic muscles whereas the capillary sprouts were chaotic and not directional in Ad-sDll4–injected ischemic muscles (Figure 5A). Furthermore, we found increased filopodia extensions from tip cells of Ad-sDll4–injected ischemic muscle vasculature (Figure 5B). Of note, Ad-sDll4–injected muscles showed many EC sprouts emerging from arterioles and disorganized accumulation of VSMCs around arterioles (Figure 5C). Thus, inhibition of Dll4-mediated signaling resulted in disordered capillary sprouting and eventually formation of nonfunctional vascular interconnections.
Figure 5. Dll4 inhibition induces sprouting angiogenesis.
A, Confocal microscopy images of adductor muscle vasculature (PECAM-1, green), (DAPI, blue) 5 days postischemia. Asterisks indicate capillary sprouts, and dotted line delimits the border of necrotic area. Scale bar: 100 μm. Left, Bar graph showing the number of capillary sprouts. *P=0.003 vs Ad-Null, n=10 mice per group. B, Confocal microscopy images showing increased filopodia extensions from tip cells of Ad-sDll4–injected ischemic muscle vasculature. Whole-mount staining for PECAM-1 (green), α-smooth muscle actin (red), and DAPI (blue). Scale bar: 50 μm. C, Confocal microscopy images showing altered arteriolar remodeling in Ad-sDll4–injected ischemic muscles. VSMCs stained with α-smooth muscle actin (red) and PECAM-1 (green). Right corner boxes, Fine confocal section of arterioles pointed by arrows. Arrowheads point newly forming myocyte fibers expressing α-smooth muscle actin. Asterisks point endothelial sprouts. Bottom, Three-dimensional reconstruction of confocal image using Volocity software shows the smooth muscle cell layer morphology of arterioles. Scale bar: 50 μm.
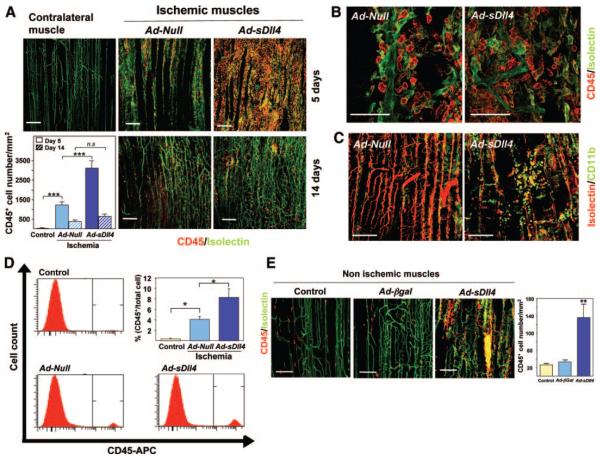
Inhibition of Dll4-Mediated Signaling Enhances Leukocyte Infiltration
Ordered leukocyte recruitment is essential to healing through participation of infiltrating leukocytes in degradation of the extracellular matrix, paracrine support of angiogenesis and removal of cellular debris. Because Notch signaling pathway is implicated in hematopoietic cell differentiation and migration, we explored the impact of Dll4 blockade on leukocyte recruitment to ischemic muscles. We found Ad-sDll4 induced a 2.5-fold increase in the number of infiltrating leukocytes (P<0.0001 versus Ad-Null), as revealed by immunofluorescence detection of cells expressing the pan leukocyte antigen CD45 and the monocyte marker CD11b (Figure 6A through 6C). Flow cytometric analysis of CD45-positive cells in single cell suspensions of muscle digests confirmed the immunohistochemical data (Figure 6D). Interestingly, in Ad-sDll4–injected muscles, leukocytes accumulated in clusters rather than remaining aligned along the vessel (Figure 6B and 6C) and showed a more abundant incorporation of bromodeoxyuridine, which suggests an increased proliferation (Online Figure IV). To determine whether the enhancement of leukocyte infiltration is attributable to a direct effect of sDll4 or is secondary to more severe ischemia, we injected Ad-sDll4 in normoperfused limb muscles. Interestingly, we found that forced sDll4 expression results in remarkable leukocyte infiltration independent of ischemia. CD45+ leukocytes accumulated in clusters and were positive for Isolectin, an indication of their activated state (Figure 6E).
Figure 6. Dll4 inhibition enhances leukocyte infiltration.
A, Confocal microscopy images and bar graph show increased number of infiltrating leukocytes in Ad-sDll4–injected muscles (CD45, green; IsolectinB4, red). Contralateral normoperfused muscles are shown for reference (control). Scale bar: 100 μm. ***P<0.0001; n=10 per group. B, High magnifications showing the linear alignment of leukocytes along the vessel long-axis in Ad-null–injected ischemic muscles and the loss of structured patterning in Ad-sDll4–injected ischemic muscles. Scale bar: 50 μm. C, Identification of CD11b leukocytes in ischemic muscles (CD11b, green; IsolectinB4, red). D, Flow cytometric analysis of single cell suspensions from skeletal muscle digests shows the increased abundance of CD45+ leukocytes in Ad-sDll4–injected muscles. *P<0.05; n=6 per group. E, Confocal images and bar graph showing induction of leukocyte infiltration in normoperfused Ad-sDll4–injected muscles (CD45, green; IsolectinB4, red). *P<0.001 vs Ad.βgal; n=6 per group.
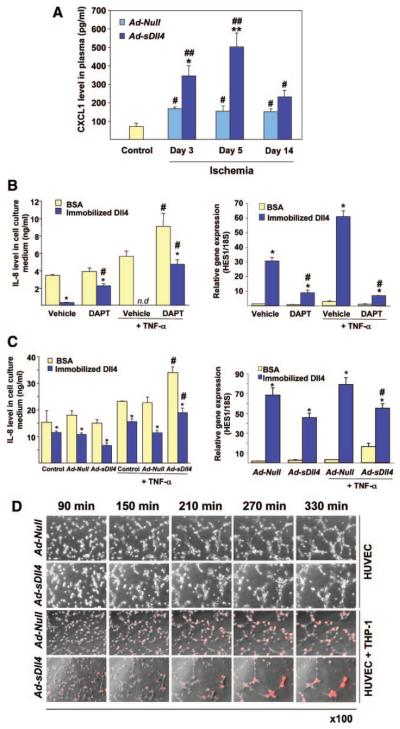
Implication of CXCL1 in Leukocyte Recruitment Under Dll4 Blockade
Next, we investigated the possibility that Dll4 blockade enhances chemokine release from ischemic muscles. Stromal cell–derived factor-1 and monocyte chemoattractant protein-1, two potent chemokines involved in monocyte recruitment, were increased in peripheral blood after ischemia but not different between Ad-Null- and Ad-sDll4–injected animals (Online Figure V). In contrast, CXCL1 levels were 2.5 to 3 fold higher in Ad-sDll4–injected mice at day 3 (P<0.05 versus Ad-Null), and day 5 (P<0.01 versus Ad-Null) postischemia (n 6 per group, Figure 7A), which corresponds to the peak of leukocyte infiltration. Infiltrating leukocytes modify the ischemic environment by releasing chemokines and growth factors. To verify whether Dll4 exerts a modulatory action on chemokines release from monocytes in an inflammatory setting, we performed in vitro assays using cultured human THP1 monocytes activated with TNF-α. Immobilized Dll4 induced Notch target gene Hes-1 and remarkably decreased the release of IL-8 (the human func- tional analog of CXCL1) by TNF-α–activated THP1 monocytes (Figure 7B). Conversely, DAPT, a γ-secretase inhibitor that blocks Notch signaling, significantly increased the IL-8 release by monocytes. Furthermore, DAPT contrasted the effect of immobilized Dll4 on Hes-1 induction and IL-8 inhibition. To verify whether the regulatory control of chemokine release by Dll4 may occur through cellular interaction, THP1 monocytes were cocultured with HUVECs transfected with Ad-sDll4 or Ad-Null (Figure 7C). The overall level of IL-8 was higher in the coculture system compared to monocyte conditioned medium as endothelial cells also synthesize IL-8. Of note, similar to DAPT, Ad-sDll4 was able to inhibit the induction of Hes-1 and to reverse the effect of immobilized Dll4 on IL-8 levels following TNF-α stimulation. Thus, Dll4 blockade may enhance IL-8 chemokine release from endothelial cells and leukocytes, thereby creating the conditions for additional recruitment of inflammatory cells.
Figure 7. Dll4 inhibition increases the release of CXCL1/IL-8.
A, Ad-sDll4 increases the circulating levels of CXCL1 in mice with limb ischemia. *P<0.05, **P*lt;0.001 vs Ad-Null; #P<0.05, **P<0.01 vs nonischemic mice (control); n=6 each group. B, Left, IL-8 concentrations in conditioned media of THP1 monocytes cultured on immobilized Dll4 in the presence of TNF-α or vehicle. To block Notch, DAPT or vehicle (DMSO) was added. Right, Hes1 expression levels in the same experiment. *P<0.001 vs BSA; #P<0.001 vs vehicle; n=4. n.d. indicates not detectable. C, Left, IL-8 concentrations in conditioned media of THP1 monocytes cocultured with transfected HUVECs. Right, Hes1 expression levels in the same experiment. *P<0.001 vs BSA; #P<0.01 vs Ad-Null; n=4. D, Time-lapse video microscopy images illustrating the endothelial network formation by HUVECs on Matrigel in the presence of DiI-labeled THP1 cells (red). HUVECs were transfected with Ad-Null or Ad-sDll4 (100 plaque-forming units/cell).
Finally, using time lapse phase-contrast video microscopy, we analyzed the effect of Ad-sDll4 on network formation by HUVECs in coculture with THP1 monocytes on Matrigel (Figure 7D and Online Movies I through IV). Under normal conditions, THP1 cells established physical contacts with HUVECs and aligned along the formed network. In contrast, under blockade of Dll4, THP1 cells were unable to spread and formed aggregates similar to those observed in vivo; furthermore, network formation was strongly inhibited. Altogether, these results indicate that a functional Dll4/Notch signaling is essential for proper recruitment of leukocytes in ischemic tissue and for interaction of leukocytes with angiogenic ECs.
Discussion
In this study, we show the upregulation of the Notch ligand Dll4 and its spatial relationship with sprouting angiogenesis in murine models of ischemia. Furthermore, we provide novel evidence for the functional importance of endogenous Dll4 in the modulation of ischemia-induced angiogenesis and control of cytokine-mediated recruitment of leukocytes.
Notch ligands have distinct roles depending on their temporal and spatial expression. Dll4 is reportedly expressed in microvessels whereas Dll1 is prevalently localized in arteries with its deficit being responsible for impaired postnatal arteriogenesis.22 We found that Dll4 is weakly expressed in normoperfused muscles but remarkably induced after ischemia. The early expression and localization of Dll4 at the level of sprouting capillaries in ischemic muscles is compatible with the possibility that Dll4 plays a role in regulating the formation of tip endothelial cells, thereby contributing to the development of a well oriented neovasculature.
To investigate the functional role of endogenous Dll4, we used a soluble form of Dll4, which blocks Dll4/Notch interaction. Effective inhibition of Notch signaling was verified by assessing the expression of target genes in in vitro assays and ischemic muscles. To dissect the impact of Dll4 blockade on neovessel sprouting, orientation, branching, and mural cell coverage, we visualized the angiogenic process at different phases of postischemic recovery using whole-mount staining of muscles combined with confocal imaging. This allowed us to have tissue-level views of the microvascular architecture and precise details on single cells and cell-cell contacts. Interestingly, Dll4 blockade resulted in the formation of a dense capillary network, which, however, appeared remarkably complex and disorganized and hence unable to supply limb muscles with adequate perfusion. This is in agreement with previous studies showing that Dll4 blockade causes functional defects in vascular development and tumor angiogenesis.9-11
To obtain deeper insight into the etiology of vascular defect, we examined the consequences of Dll4 blockade on different cellular components during early phase of reparative angiogenic process. Analysis of microvasculature at 5 days postischemia showed an enhancement of capillary sprouting in Ad-sDll4–injected muscles. Furthermore, the vascular sprouts were chaotically oriented indicating the loss of the directional program that guides nascent vascular structures to form functional interconnections. This early phase was also characterized by the disorganized accumulation of smooth muscle cells in arterioles of Ad-sDll4–injected muscles. However, we did not observe a major effect on VSMCs and pericyte coverage at 14 days postischemia, indicating that Dll4 mainly acts as a regulator of capillary angiogenesis. In line with our results, previous reports showed that VSMCs are activated by Jagged-1 and Dll1 ligands rather than Dll4.23,24
Another breakthrough of our study is the finding that blockade of Dll4/Notch interaction leads to an excessive and disorganized homing of leukocytes in ischemic muscles. Infiltrating leukocytes were positive for CD45 and CD11b, suggesting that they belong to the macrophage/monocytes lineage. Monocytes play a decisive role in supporting reparative neovascularization by multiple mechanisms. For instance, penetrating monocytes may drill tunnels in the extracellular matrix, which are subsequently colonized by endothelial sprouts, and also participate in vascular maturation.25,26 In vivo data and results from coculture of human monocytes and HUVECs indicate a crucial role of the Dll4/Notch signaling in modulating the spatial and functional association between the 2 cell types. Indeed, following in vivo Dll4 blockade, monocytes tended to aggregate in clusters rather than aligning along the vascular axis. The fact that this pattern was reproduced after injection of Ad.sDll4 in normoperfused muscles, as well as in in vitro coculture assays on Matrigel, discounts the possibility that accumulation of inflammatory cells is the consequence of a more severe ischemic damage. We found an augmented incorporation of bromodeoxyuridine by recruited cells, suggesting that stimulation of proliferation may contribute to the observed quantitative increase in infiltrating monocytes. This is agreement with studies showing that Notch ligands induce changes in growth and differentiation of myeloid cells and macrophages.27,28 We also identified a specific cytokine mechanism centered on CXCL1, which is triggered by ischemia and enhanced after Dll4 blockade. In vitro assays confirmed that IL-8 release by activated monocytes is strongly inhibited by ECs through a Dll4/Notch-mediated mechanism. In fact, we showed that this inhibitory signal could be withdrawn by Notch blockade with DAPT or sDll4. IL-8 is produced by circulating mononuclear cells and may exert, proangiogenic or proinflammatory action in a dose-dependent fashion.29,30 For instance, low dose of IL-8 induces neovascularization in experimental corneal pocket model, whereas higher doses are devoid of angiogenic activity and associated with prominent inflammatory infiltrates.31 Altogether, these data indicate that Dll4 plays an important role in restriction of inflammatory response induced by ischemia.
In conclusion, our results demonstrate that Dll4 signaling is fundamental for the formation of a functional vascular network in ischemic tissues. Moreover, Dll4/Notch signaling plays an important role in coordinating inflammation and angiogenesis to the aim of ensuring proper tissue healing.
Novelty and Significance.
What Is Known?
Delta-like (Dll)4 and its receptor, Notch, are transmembrane proteins implicated in crosstalk between neighbor cells.
Dll4 delivers inhibitory signals from tip endothelial cells (ECs) to neighboring stalk ECs limiting the formation of excessive capillary sprouts through repression of VEGFR2 (vascular endothelial growth factor receptor 2) transcription.
Blockade of Dll4/Notch interaction slows down tumor growth by interfering with maturation of tumor angiogenesis.
The involvement of Dll4/Notch interaction in reparative neovascularization after ischemia remains unexplored.
What New Information Does This Article Contribute?
Induction of myocardial ischemia and hindlimb ischemia in mice causes a remarkable upregulation of Dll4 at the forefront of sprouting capillaries.
In a model of limb ischemia, intramuscular injection of an adenovirus encoding the soluble form of Dll4 (Ad-sDll4) inhibited Notch signaling, the formation of a disorganized, low-perfused capillary network and eventually muscle degeneration.
Blockade of Dll4/Notch interaction caused remarkable infiltration of leukocytes because of repression of the Notch inhibitory control on the chemoattractant CXCL1, the functional homolog of human interleukin-8.
Dll4/Notch signaling pathway coordinates the interaction between inflammation and angiogenesis under ischemic conditions.
The role of Notch and its ligands in developmental and tumoral angiogenesis is well established. In contrast, little is known about the involvement of the Notch ligand Dll4 in reparative angiogenesis. We asked whether Dll4 could regulate proper formation of capillaries in ischemic muscles by modulation of EC sprouting and leukocyte homing. We show that Dll4 is upregulated by ischemia and that inhibition of Dll4/Notch interaction by the soluble form of Dll4 produces an exuberant, but inefficient, neovascularization responsible for tissue hypoperfusion and damage. Furthermore, we show for the first time that infiltration of ischemic muscles by leukocytes is enhanced after Dll4 blockade because of the activation of a specific cytokine mechanism centered on CXCL1 (the murine analog of human interleukin-8). Our findings elucidate a novel mechanism that coordinates vascular sprouting and inflammation in ischemia to ensure proper healing. Future studies are warranted to verify whether dysregulation of this mechanism contributes to inefficient collateralization and prolongation of inflammation in ischemic disease.
Supplementary Material
Acknowledgments
Sources of Funding
This study was funded by British Heart Foundation grant PG/06/ 035/20641.
Non-standard Abbreviations and Acronyms
- Ad
adenoviral vector
- Ad-β-Gal
adenoviral vector encoding β-galactosidase gene
- Ad-Null
adenoviral empty vector
- Ad-sDll4
adenoviral vector encoding the soluble form of Dll4 extracellular domain
- CXCL
chemokine (C-X-C motif) ligand
- DAPT
N-[N-(3,5-difluorophenacetyl-l-alanyl)]-S-phenylglycine t-butyl ester (γ secretase inhibitor)
- Dll1
Delta-like 1
- Dll4
Delta-like 4
- EC
endothelial cell
- Hes-1
hairy/enhancer of split 1
- Hey-1
hairy/enhancer of split related with YRPW motif protein 1
- HUVEC
human umbilical vein endothelial cell
- IL
interleukin
- Nrarp
Notch-regulated ankyrin repeat protein
- PCR
polymerase chain reaction
- PECAM
platelet endothelial cell adhesion molecule
- sDll4
soluble form of Delta-like 4
- THP1
human acute leukemia monocytic cell line
- TNF
tumor necrosis factor
- VSMC
vascular smooth muscle cell
Footnotes
Disclosures
None.
References
- 1.Lai EC. Notch signaling: control of cell communication and cell fate. Development. 2004;131:965–973. doi: 10.1242/dev.01074. [DOI] [PubMed] [Google Scholar]
- 2.Krebs LT, Xue Y, Norton CR, Shutter JR, Maguire M, Sundberg JP, Gallahan D, Closson V, Kitajewski J, Callahan R, Smith GH, Stark KL, Gridley T. Notch signaling is essential for vascular morphogenesis in mice. Genes Dev. 2000;14:1343–1352. [PMC free article] [PubMed] [Google Scholar]
- 3.Roca C, Adams RH. Regulation of vascular morphogenesis by Notch signaling. Genes Dev. 2007;21:2511–2524. doi: 10.1101/gad.1589207. [DOI] [PubMed] [Google Scholar]
- 4.You LR, Lin FJ, Lee CT, DeMayo FJ, Tsai MJ, Tsai SY. Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature. 2005;435:98–104. doi: 10.1038/nature03511. [DOI] [PubMed] [Google Scholar]
- 5.Siekmann AF, Lawson ND. Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature. 2007;445:781–784. doi: 10.1038/nature05577. [DOI] [PubMed] [Google Scholar]
- 6.Hellstrom M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N, Yoon K, Rossant J, Iruela-Arispe ML, Kalen M, Gerhardt H, Betsholtz C. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 7.Leslie JD, Ariza-McNaughton L, Bermange AL, McAdow R, Johnson SL, Lewis J. Endothelial signalling by the Notch ligand Delta-like 4 restricts angiogenesis. Development. 2007;134:839–844. doi: 10.1242/dev.003244. [DOI] [PubMed] [Google Scholar]
- 8.Phng LK, Gerhardt H. Angiogenesis: a team effort coordinated by notch. Dev Cell. 2009;16:196–208. doi: 10.1016/j.devcel.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 9.Li JL, Sainson RC, Shi W, Leek R, Harrington LS, Preusser M, Biswas S, Turley H, Heikamp E, Hainfellner JA, Harris AL. Delta-like 4 Notch ligand regulates tumor angiogenesis, improves tumor vascular function, and promotes tumor growth in vivo. Cancer Res. 2007;67:11244–11253. doi: 10.1158/0008-5472.CAN-07-0969. [DOI] [PubMed] [Google Scholar]
- 10.Ridgway J, Zhang G, Wu Y, Stawicki S, Liang WC, Chanthery Y, Kowalski J, Watts RJ, Callahan C, Kasman I, Singh M, Chien M, Tan C, Hongo JA, de Sauvage F, Plowman G, Yan M. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006;444:1083–1087. doi: 10.1038/nature05313. [DOI] [PubMed] [Google Scholar]
- 11.Noguera-Troise I, Daly C, Papadopoulos NJ, Coetzee S, Boland P, Gale NW, Lin HC, Yancopoulos GD, Thurston G. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature. 2006;444:1032–1037. doi: 10.1038/nature05355. [DOI] [PubMed] [Google Scholar]
- 12.Hicks C, Ladi E, Lindsell C, Hsieh JJ, Hayward SD, Collazo A, Weinmaster G. A secreted Delta1-Fc fusion protein functions both as an activator and inhibitor of Notch1 signaling. J Neurosci Res. 2002;68:655–667. doi: 10.1002/jnr.10263. [DOI] [PubMed] [Google Scholar]
- 13.Lobov IB, Renard RA, Papadopoulos N, Gale NW, Thurston G, Yancopoulos GD, Wiegand SJ. Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc Natl Acad Sci USA. 2007;104:3219–3224. doi: 10.1073/pnas.0611206104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scehnet JS, Jiang W, Kumar SR, Krasnoperov V, Trindade A, Benedito R, Djokovic D, Borges C, Ley EJ, Duarte A, Gill PS. Inhibition of Dll4-mediated signaling induces proliferation of immature vessels and results in poor tissue perfusion. Blood. 2007;109:4753–4760. doi: 10.1182/blood-2006-12-063933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Couffinhal T, Silver M, Zheng LP, Kearney M, Witzenbichler B, Isner JM. Mouse model of angiogenesis. Am J Pathol. 1998;152:1667–1679. [PMC free article] [PubMed] [Google Scholar]
- 16.Emanueli C, Zacheo A, Minasi A, Chao J, Chao L, Salis MB, Stacca T, Straino S, Capogrossi MC, Madeddu P. Adenovirus-mediated human tissue kallikrein gene delivery induces angiogenesis in normoperfused skeletal muscle. Arterioscler Thromb Vasc Biol. 2000;20:2379–2385. doi: 10.1161/01.atv.20.11.2379. [DOI] [PubMed] [Google Scholar]
- 17.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arteel GE, Thurman RG, Raleigh JA. Reductive metabolism of the hypoxia marker pimonidazole is regulated by oxygen tension independent of the pyridine nucleotide redox state. Eur J Biochem. 1998;253:743–750. doi: 10.1046/j.1432-1327.1998.2530743.x. [DOI] [PubMed] [Google Scholar]
- 19.Mailhos C, Modlich U, Lewis J, Harris A, Bicknell R, Ish-Horowicz D. Delta4, an endothelial specific notch ligand expressed at sites of physiological and tumor angiogenesis. Differentiation. 2001;69:135–144. doi: 10.1046/j.1432-0436.2001.690207.x. [DOI] [PubMed] [Google Scholar]
- 20.Benedito R, Duarte A. Expression of Dll4 during mouse embryogenesis suggests multiple developmental roles. Gene Expr Patterns. 2005;5:750–755. doi: 10.1016/j.modgep.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Landini G, Murray PI, Misson GP. Local connected fractal dimensions and lacunarity analyses of 60 degrees fluorescein angiograms. Invest Ophthalmol Vis Sci. 1995;36:2749–2755. [PubMed] [Google Scholar]
- 22.Limbourg A, Ploom M, Elligsen D, Sorensen I, Ziegelhoeffer T, Gossler A, Drexler H, Limbourg FP. Notch ligand Delta-like 1 is essential for postnatal arteriogenesis. Circ Res. 2007;100:363–371. doi: 10.1161/01.RES.0000258174.77370.2c. [DOI] [PubMed] [Google Scholar]
- 23.Sörensen I, Adams RH, Gossler A. DLL1-mediated Notch activation regulates endothelial identity in mouse fetal arteries. Blood. 2009;113:5680–5688. doi: 10.1182/blood-2008-08-174508. [DOI] [PubMed] [Google Scholar]
- 24.Doi H, Iso T, Sato H, Yamazaki M, Matsui H, Tanaka T, Manabe I, Arai M, Nagai R, Kurabayashi M. Jagged1-selective notch signaling induces smooth muscle differentiation via a RBP-Jkappa-dependent pathway. J Biol Chem. 2006;281:28555–28564. doi: 10.1074/jbc.M602749200. [DOI] [PubMed] [Google Scholar]
- 25.Arras M, Ito WD, Scholz D, Winkler B, Schaper J, Schaper W. Monocyte activation in angiogenesis and collateral growth in the rabbit hindlimb. J Clin Invest. 1998;101:40–50. doi: 10.1172/JCI119877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelly J, Ali Khan A, Yin J, Ferguson TA, Apte RS. Senescence regulates macrophage activation and angiogenic fate at sites of tissue injury in mice. J Clin Invest. 2007;117:3421–3426. doi: 10.1172/JCI32430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jonsson JI, Xiang Z, Pettersson M, Lardelli M, Nilsson G. Distinct and regulated expression of Notch receptors in hematopoietic lineages and during myeloid differentiation. Eur J Immunol. 2001;31:3240–3247. doi: 10.1002/1521-4141(200111)31:11<3240::aid-immu3240>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 28.Fung E, Tang SM, Canner JP, Morishige K, Arboleda-Velasquez JF, Cardoso AA, Carlesso N, Aster JC, Aikawa M. Delta-like 4 induces notch signaling in macrophages: implications for inflammation. Circulation. 2007;115:2948–2956. doi: 10.1161/CIRCULATIONAHA.106.675462. [DOI] [PubMed] [Google Scholar]
- 29.Barcelos LS, Duplaa C, Krankel N, Graiani G, Invernici G, Katare R, Siragusa M, Meloni M, Campesi I, Monica M, Simm A, Campagnolo P, Mangialardi G, Stevanato L, Alessandri G, Emanueli C, Madeddu P. Human CD133+ progenitor cells promote the healing of diabetic ischemic ulcers by paracrine stimulation of angiogenesis and activation of Wnt signaling. Circ Res. 2009;104:1095–1102. doi: 10.1161/CIRCRESAHA.108.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM, Elner SG, Strieter RM. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992;258:1798–1801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- 31.Strieter RM, Kunkel SL, Elner VM, Martonyi CL, Koch AE, Polverini PJ, Elner SG. Interleukin-8. A corneal factor that induces neovascularization. Am J Pathol. 1992;141:1279–1284. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.