Figure 1.
Foxp2-R552H Mutant Mice
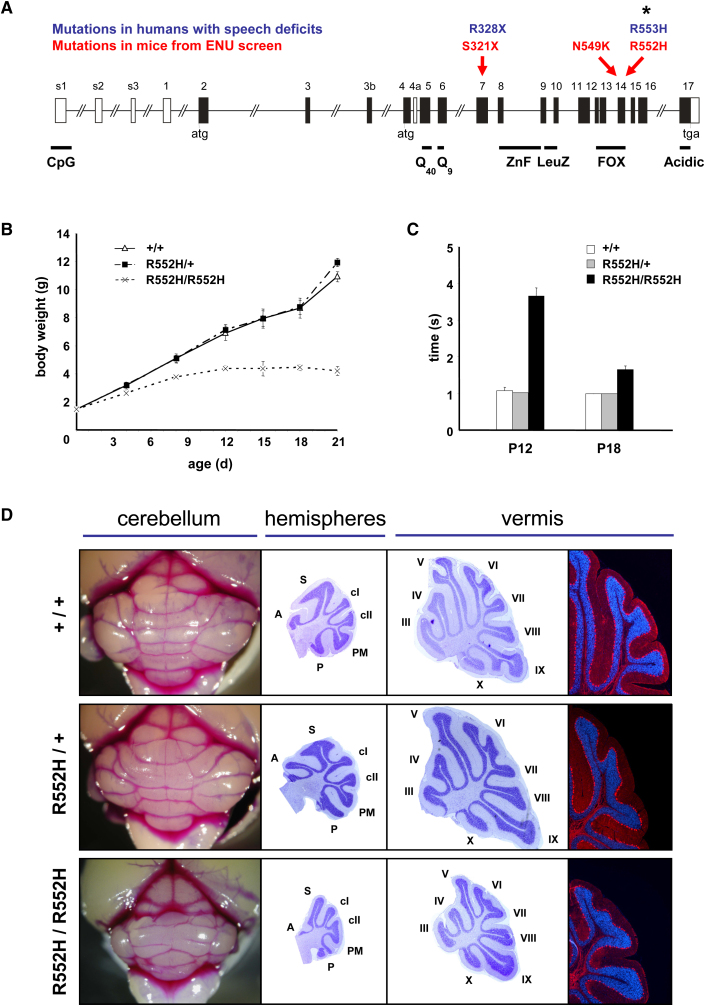
(A) Allelic series for mouse Foxp2. Mutations from gene-driven ENU mutagenesis screens are shown above a schematic of the murine Foxp2 locus, spanning more than 500 kb of genomic DNA. Superimposed are FOXP2 point mutations found in humans with speech and language disorders. Asterisk indicates the KE family mutation. Murine Foxp2 is one amino acid shorter than human FOXP2 because of a shorter polyglutamine tract (Q9 instead of Q10); hence, R552H in mice corresponds to R553H in humans.
(B) Time course of postnatal bodyweight development. Homozygous R552H mice (n = 5) show a strongly reduced weight gain, despite feeding normally and receiving similar maternal care to littermates. Development of heterozygotes (n = 13) is indistinguishable from that of wild-type littermates (n = 8) (mean ± standard error of the mean [SEM]).
(C) Postnatal righting-reflex development. Homozygous R552H mice (n = 8) display a significantly delayed righting reflex. Heterozygotes (n = 15) are indistinguishable from wild-type littermates (n = 4) (mean ± SEM).
(D) Cerebellar morphology at postnatal day 21 in wild-type (top row), heterozygous (middle), and homozygous R552H mice (bottom). Homozygotes display reduced cerebellar size (left-hand column) and foliation deficits in hemispheres and vermis (middle columns, cresyl violet staining). Nevertheless, Purkinje cells are aligned in a monolayer (right-hand column) as revealed by anti-calbindin immunohistochemistry (red) and DAPI nuclear staining (blue). Heterozygotes show no detectable alterations in cerebellar size, foliation, or layering. Vermis lobules are labeled III-X; hemispheric lobules are anterior (A), simplex (S), crus I (cI), crus II (cII), paramedian (PM), and pyramidis (P). All photographs taken at the same magnification. Concordant findings from other mutations of the allelic series are shown in Figures S1–S4.