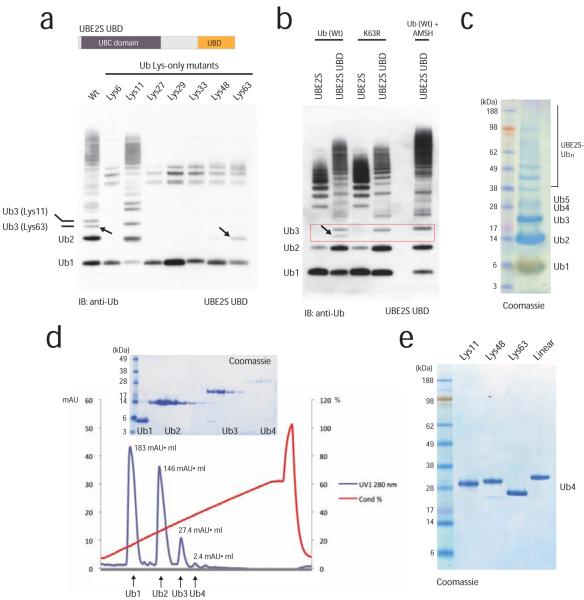
Figure 3.
Assembly of Lys11-linked tetraubiquitin
(a) UBE2S engineering to increase yields of free Lys11-linked ubiquitin chains. The C-terminal tail was replaced with the ZnF-UBP domain of USP5/IsoT. The fusion protein assembles free chains of up to five ubiquitin molecules, yet it is less specific and also incorporates Lys63-linkages with wild-type and Lys63-only ubiquitin (indicated by arrows). (b) Incorporation of Lys63-linkages can be counteracted by using a K63R ubiquitin mutant, or by including the Lys63-specific DUB AMSH in the reaction, as observed by disappearance of the faster migrating Lys63-linkage contamination. (c) 5 μl aliquot of a 1 ml chain assembly reaction using 25 mg ubiquitin shows that di-, tri- and tetraubiquitin is generated in milligram quantities. (d) Cation exchange chromatography was used to purify Lys11-linked ubiquitin chains. The integrated peak area (mAU*ml) is specified. A gel showing protein-containing fractions is shown as an inset. (e) Purified ubiquitin tetramers of Lys11, Lys48, Lys63 and linear linkages have different electrophoretic mobility on 4-12% SDS-PAGE gels.