Abstract
G protein-coupled receptors (GPCRs) are important molecular targets in drug discovery. These receptors play a pivotal role in physiological signaling pathways and are targeted by nearly 50% of currently available drugs. Mounting evidence suggests that GPCRs form dimers and various studies have shown that dimerization is necessary for receptor maturation, signaling and trafficking. However, the physiological implications of dimerization in vivo have not been well explored since detection of GPCR dimers in endogenous systems has been a challenging task. One exciting new approach to this challenge is the generation of antibodies against specific GPCR dimers. Such antibodies could be used as tools for characterization of heteromer-specific function, as reagents for their purification, tissue localization and regulation in vivo and as probes for mapping their functional domains. In addition, such antibodies could serve as alternative ligands for GPCR heteromers. Thus, heteromer-specific antibodies represent novel tools for the exploration and manipulation of GPCR dimer pharmacology.
Keywords: G protein-coupled receptors, GPCRs, dimers, heteromers, antibodies
G protein-coupled receptors (GPCRs) are a family of integral membrane proteins located on the cell surface, whose activation induces second messenger amplification. These receptors are drug targets in a wide range of major diseases such as asthma, cancer, diabetes, hypertension, obesity, chronic pain, and other neurological disorders.
[Call-out] Located on the cell surface, G protein-coupled receptors (GPCRs) are a family of integral membrane proteins that are drug targets in a wide range of major diseases such as asthma, cancer, diabetes, hypertension, obesity, chronic pain, and other neurological disorders.
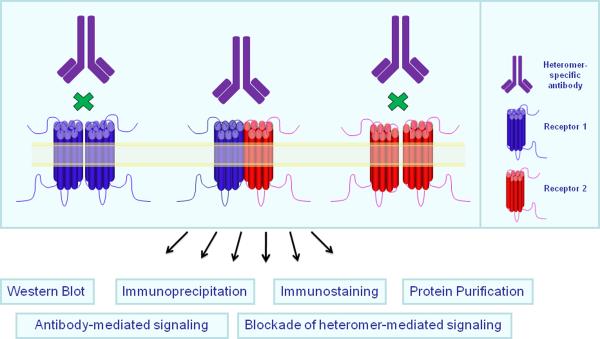
For a number of years it was thought that individual GPCRs functioned as a monomeric unit. However, in recent years several studies showed that GPCRs can form dimers such as homo- and heteromers as well as more complex oligomers (1–3). There is also growing evidence suggesting that dimerization substantially modulates receptor function (4). Studies have shown that receptor heteromers have important roles in receptor maturation, signaling and trafficking (5–8). Most of these studies were carried out in heterologous cell systems over-expressing different epitope tagged GPCRs using a variety of assays including ligand binding, coimmunoprecipitation, (9–10), Bioluminescence Resonance Energy Transfer (BRET) (11–13) or Fluorescence Resonance Energy Transfer (FRET) (14–16). However, detection and characterization of specific GPCR dimers in an endogenous system has been challenging due to the lack of selective tools. We propose the generation of GPCR dimer-specific antibodies that could be used as tools for the characterization of heteromer pharmacology and signaling, and as reagents for the study of their in vivo localization and regulation. In addition, such antibodies could be developed as alternative highly selective ligands targeting GPCR heteromers (Figure 1); the functional and physiological diversity of GPCR dimers provides the necessary targets for enabling novel drug discovery.
Figure 1.
A monoclonal antibody can be generated that specifically recognizes receptor heteromers, but not homomers. It can be used as a tool to detect receptor heteromers in vivo and to characterize heteromer-specific signaling.
In this review, we focus our attention on the potential implications of antibodies in the development of selective reagents targeting GPCR dimers.
G PROTEIN-COUPLED RECEPTORR DIMERIZATION AND ALLOSTERISM
It is now well established that GPCRs exist and function as dimers/oligomers. Interactions between identical protomers (a single GPCR) are referred to as homomers and interactions between nonidentical protomers are referred to as heteromers. Additionally, there is growing evidence that heteromerization can generate receptors with novel characteristics, leading to altered pharmacological properties (1, 17–18).
[Callout] It is now well established that GPCRs exist and function as dimers/oligomers. Interactions between identical protomers (a single GPCR) are referred to as homomers and interactions between nonidentical protomers are referred to as heteromers. Additionally, there is growing evidence that heteromerization can generate receptors with novel characteristics, leading to altered pharmacological properties
The most convincing evidence for receptor heteromerization has come from studies with metabotropic GABAB receptors in heterologous systems where the functional receptor requires the co-expression of both the GABABR1 and GABABR2 subunits (19). In the absence of GABABR2, the GABABR1 subunit is retained in the endoplasmic reticulum while in the absence of GABABR1, the GABABR2 subunit is expressed at the cell surface but is non-functional; only the co-expression of the two subunits leads to plasma membrane expression of a functional receptor. Heteromerization leads to the masking of an ER retention sequence in GABAB1 by GABAB2, allowing the heterodimers to be efficiently expressed at the cell surface (20,21). This kind of assosciation is found to occur very shortly after biosynthesis, meaning that dimerization could be key to receptor maturation in the case of this and other GPCRs (7, 21–22).
The concept that GPCR heteromerization could have a role in pharmacological diversity was first observed in studies on the δ- and κ-opioid receptors (17). These studies show that the δ-κ complex exhibits ligand binding, functional and trafficking properties that are distinct from those of each individual receptor (17). Studies also show that μ and δ receptors, once thought to only function individually, can dimerize and form a complex exhibiting distinct functional properties (23) that have profound effects on morphine mediated analgesia. It is probable that additional pairings involving the μ-opioid receptor and other GPCRs could play crucial roles in regulating analgesia and addiction (24). In another compelling case, the mammalian ability to perceive sweet tastes depends on the heteromerization of T1R2 and T1R3 taste receptors whereas the umami taste relies on a T1R1/T1R3 dimer, indicating that heterodimerization between different subtypes of the taste receptor leads to modulation of ligand binding properties resulting in the recognition of very distinctive tastes (25). Studies also show that receptors thought to be normally involved in completely different physiological systems may form heterodimers (26) thus allowing for a new level of specificity for drug targeting.
In some cases, GPCR dimerization/heterodimerization is associated with changes in ligand binding affinity for the complex compared to the affinity for the homomer implying heteromer-mediated modulation of the ligand binding pocket. Several studies have shown positive or negative co-operativity in ligand binding following receptor co-expression likely resulting from receptor heterodimerization. Examples include GPCR dimer pairs such as muscarinic m2/m3 (27), somatostatin SSTR5/dopamine D2 (15), adenosine A2A/dopamine D1 (28) and the opioid δ/μ (23, 29) receptors. These results suggest that heteromerization between pharmacologically different receptors provides a level of diversity that could generate new opportunities for the development of more selective compounds that would target specific heterodimers without affecting the activity of individual protomers.
In an attempt to develop drugs that target GPCR heterodimers bivalent ligands have been developed (30). Bivalent ligands are composed of two functional pharmacophores linked by a spacer that bind to the orthostheric sites of the protomers within a heteromer. In principle, bivalent ligands would preferentially bind to the heteromer, the latter is likely to be selectively expressed only in tissues co-expressing both of the interacting receptors, and this would lead to improved drug targeting and tissue selectivity. Some bivalent ligands were found to be effective in increasing the specificity of drug effects i.e. increased analgesia with a reduction in side-effects such as development of tolerance suggesting that bivalent ligands could be developed as potential novel analgesics (31–32). A set of such ligands targeted both μ and δ receptors and consisted of a μ agonist pharmacophore (oxymorphone) and a δ antagonist pharmacophore (NTI) separated by spacers of variable lengths (33). The antinociceptive activity of these bivalent ligands was reported to be better than that achieved by co-administration of the individual pharmacophores and could be titrated by manipulating the distance between the pharmacophores (33). Another group of bivalent ligands targeting δ-κ heteromers were recently synthesized consisting of δ and κ antagonists (NTI and 5'-GNTI, respectively) linked by variable length spacers (34). One these compounds (KDN-21) exhibited a much higher affinity and selectivity for δ-κ heterodimers relative to δ or κ receptors alone (35). KDN-21 was found to have antinociceptive activity when administered directly into the spinal cord, but not into the brain supporting the notion that the relative levels of a heterodimer vary in different regions and that selective targeting a heterodimer could increase the specificity of a drug with probable reduction of associated side-effects. Taken together, these studies show that bivalent ligands to opioid receptors could be developed as novel analgesics with greater tissue selectivity and lesser degree of side-effects normally associated with chronic opiate administration.
The possibility of allosterically modulating the function of individual protomers within a GPCR dimer makes the heteromer a new type of drug target. Classically, the majority of drugs target the receptor's orthosteric site that is often the binding site for the endogenous agonist. Recent studies have begun to explore the possibility of targeting allosteric sites and have identified synthetic allosteric modulators (activator or inhibitors of GPCRs) (36, 37). These alternate sites can bind small molecule ligands leading to the modulation of agonist binding to the orthosteric site. In the case of a GPCR dimer, a ligand bound to the orthosteric site of one protomer could function as an allosteric modulator of the other protomer within the pair, which could potentially alter the conformation and functional outcome of either protomer, receptor heteromer and/or the whole complex. The design of such drugs would help us understand the unique pharmacology and signaling mechanisms activated by GPCR dimers given the mounting evidence suggesting that every GPCR dimer displays unique functional properties with different pharmacological characteristics (15, 27–29). It is likely that heteromerization produces a unique binding pocket that binds ligands with distinct affinity and display unique ligand structure-activity relationships compared to receptor homomers. Thus it would be advantageous to begin to find smaller, drug-like synthetic compounds that would bind to allosteric sites in either protomer and alter the conformation of a dimer's orthostheric binding pocket. These smaller, single-target drugs might also be made to target specific sites in the novel binding site created in the pocket between the two interacting receptor proteins. In this context, dimer-selective antibodies would be useful agents for the exploration of dimerization within in vitro as well as in vivo systems.
One of the reasons why GPCR dimers are exciting drug targets is that a change in their expression levels could contribute to the development of disease symptoms in specific types of tissues.
[Callout] One of the reasons why GPCR dimers are exciting drug targets is that a change in their expression levels could contribute to the development of disease symptoms in specific types of tissues.
For example, it has been reported that preeclamptic hypertensive women exhibit a significant increase in heteromers between the AT1 angiotensin receptor (that responds to the vasopressor angiotensin II), and the B2 bradykinin receptor (that responds the vasodepressor bradykinin). AT1 and B2 bradykinin receptors normally regulate blood pressure by controlling vasoconstriction and smooth muscle relaxation, respectively. During preeclampsia the dimeric AT1-B2 receptor complex becomes hypersensitive to angiotensin II and exhibits reduced affinity for the B2 receptor agonist, bradykinin (38). This example of in vivo alteration of GPCR pharmacology by dimerization indicates that dimers could be useful for controlling blood pressure. Disease-specific GPCR dimers are also thought to play roles in the regulation of cardiac muscle cell function, asthma, schizophrenia, drug-related analgesia and tolerance, and other pathologies (23, 39– 42). However, the lack of suitable to tools to study the distribution and regulation of heteromers in vivo has made such studies difficult. Recent advances in antibody technology have begun to help address some of these difficult questions; these are described below.
[Callout] The lack of suitable tools to study the distribution and regulation of heteromers in vivo has made the study of disease-specific GPCR dimers difficult. Recent advances in antibody technology have begun to help address some of these difficult questions.
ANTIBODIES AS G PROTEIN-COUPLED RECEPTOR DRUG TARGETS
Antibodies have been used as tools for receptor characterization, purification, localization and as probes for mapping their functional domains. Antibodies are now becoming integral tools in drug research and are even being developed as drugs. Their unique design makes them especially suited for attaining a high level of specificity for a large variety of organic, pharmacologically significant molecules and epitopes on larger molecules. In particular, monoclonal antibodies which by definition recognize a single epitope are quite useful in these applications, unlike polyclonal antibodies which target multiple epitopes.
Antibodies are excellent diagnostic screening tools as they can detect the domains involved in activity-mediated conformational changes of signaling proteins, including receptors (43– 51).
[Callout] Antibodies are excellent diagnostic screening tools as they can detect the domains involved in activity-mediated conformational changes of signaling proteins, including receptors.
For example, a monoclonal antibody to the N-terminal region of rhodopsin exhibited a higher degree of recognition for activated receptors than for inactivated receptors even after detergent treatment, suggesting that photoactivation of rhodopsin induces a conformational change at the N-terminus that exposes an epitope that is recognized by the monoclonal antibody (52). Recently, Gupta et al. (53, 54) showed that antibodies targeting the N-termini of family A GPCR homomers can discriminate between activation states of the receptors. Since the extent to which a certain antibody binds to a receptor can depend on whether the latter is activated by a functional ligand, an assay using antibodies could be used to effectively screen for novel GPCR ligands. This method recently led to the identification of hemopressin as an interesting new peptide antagonist of the CB1 cannabinoid receptor (55). In addition, it will be important to develop heteromer-specific antibodies, given the critical role of receptor heteromers in certain cell signaling and disease processes.
Several studies have shown that antibodies directed against GPCRs can act as allosteric receptor agonists or antagonists (56–59).
[Callout] Several studies have shown that antibodies directed against GPCRs can act as allosteric receptor agonists or antagonists.
In the case of δ opioid receptor, an antibody directed to the N-terminal region behaves like a classic agonist and activates the receptor (58). For the β–adrenergic receptor, a monoclonal antibody to the second extracellular loop had agonist-like activity, while a monovalent (Fab) fragment acted as an antagonist (59). Antibodies are usually very effective when they target the extracellular N- and C-termini or the third intracellular loop of GPCRs (53, 60). Because these regions are highly diverse, the problem of antibodies binding to similarly structured molecules, which plagues synthetic drugs, is practically eliminated.
Antibodies can also be useful in examining the conformational changes of a particular protomer that arise upon binding of a ligand to the partner protomer (57).
[Callout Antibodies can also be useful in examining the conformational changes of a particular protomer that arise upon binding of a ligand to the partner protomer.
For example, an antibody binding to an allosteric site on one protomer could differentially detect whether one or both protomers are bound by selective orthosteric/allosteric ligands (57). This could be used as a screening strategy for the detection of novel allosteric ligands targeting heteromers. Allosteric drugs could be useful because they leave the main active site free, only modulating its behavior. The side-effects associated with modern orthosteric drugs are often due to the replacement of the receptor's endogenous ligand, altering homeostasis far more than is necessary. Thus, using antibodies as allosteric drugs could circumvent this problem.
Antibodies, including humanized antibodies, are currently being used to detect receptors, their activity states, and their location and density in the treatment of immune system diseases and cancers (61).
[Callout] Antibodies, including humanized antibodies, are currently being used to detect receptors, their activity states, and their location and density in the treatment of immune system diseases and cancers.
As already mentioned, antibodies can act as functional modulators, direct agonists, inverse agonists and antagonists. In addition, antibodies can be designed to carry marker molecules that bind to a selective secondary drug, or they may simply be designed to carry a toxin, for example, to kill target cancer cells (62). This way, only cells expressing certain receptors or specific receptor dimers would be targeted and altered or destroyed. Currently, 22 monoclonal antibody drugs have been approved by the FDA for therapeutic use, while hundreds more are undergoing clinical trials. Other than vaccines, antibodies are the fastest growing drug category and have the potential to bring under control a vast array of disorders (62).
CONCLUSION
Mounting evidence suggests that GPCR dimerization is an important physiological method of restoring equilibrium. It is easy to see that dimer regulation is uniquely useful in the body and that understanding dimer pharmacology could lead to the development of far more selective drugs and to more effective treatments for diseases. Thus, antibodies against G-protein-coupled receptor dimers could be useful tools in detection of receptor dimers, in assessment of dimer pharmacology, in screening during drug development. In the near future, antibodies could be generated and optimized to serve as drugs by directly targeting tissues that exhibit altered levels of GPCR dimers and selectively block or activate heteromer function.
Acknowledgements
This work was supported by NIH grants DA08862, DA19521 and 1P50GM071558-01A27398 (SBCNY) to Lakshmi A. Devi. Ittai Bushlin is a trainee in the Integrated Pharmacological Sciences Training Program supported by grant T32GM062754 from the National Institute of General Medical Sciences.
REFERENCES
- 1.Bouvier M. Oligomerization of G-protein-coupled transmitter receptors. Nat Rev Neurosc. 2001;2(4):274–286. doi: 10.1038/35067575. [DOI] [PubMed] [Google Scholar]
- 2.Angers S, Salahpour A, Bouvier M. Dimerization: an emerging concept for G-protein-coupled receptor ontogeny and function. Annu Rev Pharmacol Toxicol. 2002;42:409–435. doi: 10.1146/annurev.pharmtox.42.091701.082314. [DOI] [PubMed] [Google Scholar]
- 3.George SR, O'Dowd BF, Lee SP. G-protein-coupled receptor oligomerization and its potential for drug discovery. Nat Rev Drug Discov. 2002;1(10):808–20. doi: 10.1038/nrd913. [DOI] [PubMed] [Google Scholar]
- 4.Milligan G. Gprotein-coupled receptor dimerization:Function and ligand pharmacology. Mol Pharmacol. 2004;66(1):1–7. doi: 10.1124/mol.104.000497.. [DOI] [PubMed] [Google Scholar]
- 5.Devi LA. G-protein-coupled receptor dimers in the lime light. Trends Pharmacol Sci. 2000;21(9):324–326. doi: 10.1016/s0165-6147(00)01519-4. [DOI] [PubMed] [Google Scholar]
- 6.Jordan BA, Trapaidze N, Gomes I, Nivarthi R, Devi LA. Dimerization of opioid receptors with β2adrenergic receptors: a role in trafficking and mitogen activated protein kinase activation. Proc Natl Acad Sci USA. 2001;98:343–348. doi: 10.1073/pnas.011384898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terrillon S, Bouvier M. Roles of G-protein-coupled receptor dimerization. EMBO Rep. 2004;5(1):30–34. doi: 10.1038/sj.embor.7400052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bulenger S, Marullo S, Bouvier M. Emerging role of homo- and heterodimerization in G-protein-coupled receptor biosynthesis and maturation. Trends Pharmacol Sci. 2005;26(3):131–137. doi: 10.1016/j.tips.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Hebert TE, Moffett S, Morello JP, Loisel TP, Bichet DG, Barret C, Bouvier M. A peptide derived from a β2-adrenergic receptor transmembrane domain inhibits both receptor dimerization and activation. J Biol Chem. 1996;271:16384–16392. doi: 10.1074/jbc.271.27.16384. [DOI] [PubMed] [Google Scholar]
- 10.Cvejic S, Devi LA. Dimerization of the δ opioid receptor: implication for a role in receptor internalization. J Biol Chem. 1997;272:26959–26964. doi: 10.1074/jbc.272.43.26959. [DOI] [PubMed] [Google Scholar]
- 11.Angers S, Salahpour A, Joly E, Hilairet S, Chelsky D, Dennis M, Bouvier M. Detection of beta 2-adrenergic receptor dimerization in living cells using bioluminescence resonance energy transfer (BRET) Proc Natl Acad Sci U S A. 2000;28; 97(7):3684–3689. doi: 10.1073/pnas.060590697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McVey M, Ramsay D, Kellett E, Rees S, Wilson S, Pope AJ, Milligan G. Monitoring receptor oligomerization using time-resolved fluorescence resonance energy transfer and bioluminescence resonance energy transfer. The human delta -opioid receptor displays constitutive oligomerization at the cell surface, which is not regulated by receptor occupancy. J Biol Chem. 2001;276:14092–14099. doi: 10.1074/jbc.M008902200. [DOI] [PubMed] [Google Scholar]
- 13.Ayoub MA, Couturier C, Lucas-Meunier E, Angers S, Fossier P, Bouvier M, Jockers R. Monitoring of ligand-independent dimerization and ligand-induced conformational changes of melatonin receptors in living cells by bioluminescence resonance energy transfer. J Biol Chem. 2002;277:21522–21528. doi: 10.1074/jbc.M200729200. [DOI] [PubMed] [Google Scholar]
- 14.Overton MC, Blumer KJ. G-protein coupled receptors function as oligomers in vivo. Curr Biol. 2000;10:341–344. doi: 10.1016/s0960-9822(00)00386-9. [DOI] [PubMed] [Google Scholar]
- 15.Rocheville M, Lange DC, Kumar U, Sasi R, Patel RC, Patel YC. Receptors for dopamine and somatostatin: formation of hetero-oligomers with enhanced functional activity. Science. 2000a;288:154–157. doi: 10.1126/science.288.5463.154. [DOI] [PubMed] [Google Scholar]
- 16.Dinger MC, Bader JE, Kobor AD, Kretzschmar AK, Beck-Sickinger AG. Homodimerization of neuropeptide Y receptors investigated by fluorescence resonance energy transfer in living cells. J Biol Chem. 2003;278:10562–10571. doi: 10.1074/jbc.M205747200. [DOI] [PubMed] [Google Scholar]
- 17.Jordan BA, Devi LA. G-protein-coupled receptor heterodimerization modulates receptor function. Nature. 1999;399:697–700. doi: 10.1038/21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devi LA. Heterodimerization of G-protein-coupled receptors: pharmacology, signaling and trafficking. Trends Pharmacol Sci. 2001;22(10):532–537. doi: 10.1016/s0165-6147(00)01799-5. [DOI] [PubMed] [Google Scholar]
- 19.Jones KA, Borowski B, Tamm JA, Craig DA, Durkin MM, Dai M, Yao WJ, Johnson M, Gunwaldsen C, Huang LY, Tang C, Shen Q, Salon JA, Morse K, Laz T, Smith KE, Nagarathnam D, Noble SA, Branchek TA, Gerald C. GABAB receptor function as a heterotrimeric assembly of the subunits GABABR1 and GABABR2. Nature. 1998;396:674–679. doi: 10.1038/25348. [DOI] [PubMed] [Google Scholar]
- 20.Pin JP, Kniazeff J, Liu J, Binet V, Goudet C, Rondard P, Prézeau L. Allosteric functioning of dimeric class G-protein-coupled receptors. FEBS J. 2005;272(12):2947–2955. doi: 10.1111/j.1742-4658.2005.04728.x. [DOI] [PubMed] [Google Scholar]
- 21.Galvez T, Duthey B, Kniazeff J, Blahos J, Rovelli G, Bettler B, Prezeau L, Pin JP. Allosteric interactions between GB1 and GB2 subunits are required for optimal GABAB receptor function. EMBO J. 2001;20:2152–2155. doi: 10.1093/emboj/20.9.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marshall FH, Jones KA, Kaupmann K, Bettler B. GABAB receptors – the first 7TM heterodimers. Trends Pharmacol Sci. 1999;20:396–399. doi: 10.1016/s0165-6147(99)01383-8. [DOI] [PubMed] [Google Scholar]
- 23.Gomes I, Gupta A, Filipovska J, Szeto HH, Pintar JE, Devi LA. A role of heterodimerization of mu and delta opiate receptors in enhancing morphine analgesia. Proc Natl Acad Sci U S A. 2004;101(14):5135–5139. doi: 10.1073/pnas.0307601101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rios C, Gomes I, Devi LA. Mu opioid and CB1 cannabinoid receptor interactions: reciprocal inhibition of receptor signaling and neuritogenesis. Br J Pharmacol. 2006;148(4):387–395. doi: 10.1038/sj.bjp.0706757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E. Human receptors for sweet and umami taste. Proc Natl Acad Sc USA. 2002;99:4692–4696. doi: 10.1073/pnas.072090199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mei B. Dimerization of G-protein-coupled receptors: roles in signal transduction. Cellular Signalling. 2004;16:175–186. doi: 10.1016/s0898-6568(03)00128-1. [DOI] [PubMed] [Google Scholar]
- 27.Maggio R, Barbier P, Colelli A, Salvadori F, Demontis G, Corsini GU. G-protein-linked receptors: pharmacological evidence for the formation of heterodimers. J Pharmacol Exp Ther. 1999;291(1):251–257. [PubMed] [Google Scholar]
- 28.Franco R, Ferre S, Agnati L, Torvinen M, Gines S, Hillion J, Casado V, Lledo PM, Zoll M, Lluis C, Fuxe K. Evidence for adenosine/dopamine receptor interactions: indications for heteromerization. Neuropsychopharmacol. 2000;23:S50–S59. doi: 10.1016/S0893-133X(00)00144-5. [DOI] [PubMed] [Google Scholar]
- 29.Gomes I, Jordan BA, Gupta A, Trapaidze N, Nagy V, Devi LA. Heterodimerization of mu and delta opioid receptors: a role in opiate synergy. J Neurosci. 2000;15,20(22):RC110. doi: 10.1523/JNEUROSCI.20-22-j0007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berque-Bestel I, Lezoualc'h F, Jockers R. Bivalent ligands as specific pharmacological tools for G protein-coupled receptor dimers. Curr Drug Discov Technol. 2008;5(4):312–318. doi: 10.2174/157016308786733591. [DOI] [PubMed] [Google Scholar]
- 31.Ballet S, Pietsch M, Abell AD. Multiple ligands in opioid research. Protein Pept Lett. 2008;15(7):668–682. doi: 10.2174/092986608785133672. [DOI] [PubMed] [Google Scholar]
- 32.Gupta A, Decaillot FM, Devi LA. Targeting opioid receptor heterodimers: strategies for screening and drug development. AAPS J. 2006;10,8(1):E153–E159. doi: 10.1208/aapsj080118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daniels DJ, Lenard NR, Etienne CL, Law PY, Roerig SC, Portoghese PS. Opioid-induced tolerance and dependence in mice is modulated by the distance between pharmacophores in a bivalent ligand series. Proc Natl Acad Sci U S A. 2005;102(52):19208–19213. doi: 10.1073/pnas.0506627102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhushan RG, Sharma SK, Xie Z, Daniels DJ, Portoghese PS. A bivalent ligand (KDN-21) reveals spinal delta and kappa opioid receptors are organized as heterodimers that give rise to delta(1) and kappa(2) phenotypes. Selective targeting of delta-kappa heterodimers. J Med Chem. 2004;47(12):2969–2972. doi: 10.1021/jm0342358. [DOI] [PubMed] [Google Scholar]
- 35.Xie Z, Bhushan RG, Daniels DJ, Portoghese PS. Interaction of bivalent ligand KDN21 with heterodimeric delta-kappa opioid receptors in human embryonic kidney 293 cells. Mol Pharmacol. 2005;68(4):1079–1086. doi: 10.1124/mol.105.012070. [DOI] [PubMed] [Google Scholar]
- 36.Brady AE, Jones CK, Bridges TM, Kennedy JP, Thompson AD, Heiman Ju, Breininger ML, Gentry PR, Yin H, Jadhav SB, Shirey JK, Conn PJ, Lindsley CW. Centrally active allosteric potentiators of the M4 muscarinic acetylcholine receptor reverse amphetamine-induced hyperlocomotor activity in rats. J Pharmacol Exp Ther. 2008;327(3):941–953. doi: 10.1124/jpet.108.140350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang L, Martin B, Brenneman R, Luttrell LM, Maudsley S. Allosteric modulators of G-protein-coupled receptors: future therapeutics for complex physiological disorders. J Pharmacol Exp Ther. 2009;331(2):340–348. doi: 10.1124/jpet.109.156380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.AbdAlla S, Lother H, el Massiery A, Quitterer U. Increased AT(1) receptor heterodimers in preeclampsia mediate enhanced angiotensin ii responsiveness. Nat Med. 2001;7(9):1003–1009. doi: 10.1038/nm0901-1003. [DOI] [PubMed] [Google Scholar]
- 39.Barki-Harrington L, Luttrell LM, Rockman HA. Dual inhibition of betaadrenergic and angiotensin II receptors by a single antagonist: a functional role for receptor-receptor interaction in vivo. Nature Medicine. 2003;108:1611–1618. doi: 10.1161/01.CIR.0000092166.30360.78. [DOI] [PubMed] [Google Scholar]
- 40.McGraw DW, Mihlbachler KA, Schwarb MR, Rahman FF, Small KM, Almoosa KF, Liggett SB. Airway smooth muscle prostaglandin-EP1 receptors directly modulate beta2-adrenergic receptors within a unique heterodimeric complex. J Clin Invest. 2006;116:1400–1409. doi: 10.1172/JCI25840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abul-Husn NS, Sutak M, Milne B, Jhamandas K. Augmentation of spinal morphine analgesia an inhibition of tolerance by low doses of mu- and delta-opioid receptor antagonists. Br J Pharmacol. 2007;151:877–887. doi: 10.1038/sj.bjp.0707277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gonzalez-Maeso J, Ang RL, Yuen T, Chan P, Weisstanb NV, Lopez-Gimenez JF, Zhou H, Okawa Y, Callado LF, Milligen G, Gingrich JA, Filizola M, Meana JJ, Sealfon SC. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature. 2008;452:93–97. doi: 10.1038/nature06612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmidt A, MacColl R, Lindau-Shepard B, Buckler DR, Dias JA. Hormone-induced conformational change of the purified soluble hormone binding domain of follitropin receptor complexed with single chain follitropin. J Biol Chem. 2001;276:23373–23381. doi: 10.1074/jbc.M100057200. [DOI] [PubMed] [Google Scholar]
- 44.Vignal E, Blangy A, Martin M, Gauthier-Rouviere C, Fort P. Kinectin is a key effector of RhoG microtubule-dependent cellular activity. Mol Cell Biol. 2001;21:8022–8034 A. doi: 10.1128/MCB.21.23.8022-8034.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu J, Chuntharapai A, Beck J, Bass S, Ow A, De Vos AM, Gibbs V, Kim KJ. Structure-Function study of the extracellular domain of the human IFN-{alpha} receptor (hIFNAR1) using blocking monoclonal antibodies: The role of domains 1 and 21. J Immunol. 1998;160:1782–1788. [PubMed] [Google Scholar]
- 46.Morgan EL, Ember JA, Sanderson SD, Scholz W, Buchner R, Ye RD, Hugli TE. Anti-C5a receptor antibodies. Characterization of neutralizing antibodies specific for a peptide, C5aR-(9–29), derived from the predicted amino-terminal sequence of the human C5a receptor. J Immunol. 1993;151:377–388. [PubMed] [Google Scholar]
- 47.Lebesgue D, Wallukat G, Mijares A, Granier C, Argibay J, Hoebeke J. An agonist-like monoclonal antibody against the human beta(2)-adrenoceptor. Eur J Pharmacol. 1998;348:123–133. doi: 10.1016/s0014-2999(98)00136-8. [DOI] [PubMed] [Google Scholar]
- 48.Salle L, Eftekhari P, Aupart M, Cosnay P, Hoebeke J, Argibay JA. Inhibitory activity of antibodies against the human atrial 5-HT receptor. J Mol Cell Cardiol. 2001;33:405–417. doi: 10.1006/jmcc.2000.1312. [DOI] [PubMed] [Google Scholar]
- 49.Blanpain C, Vanderwinden JM, Cihak J, Wittamer V, Le Poul E, Issafras H, Stangassinger M, Vassart G, Marullo S, Schlndorff D, Parmentier M, Mack M. Multiple active states and oligomerization of CCR5 revealed by functional properties of monoclonal antibodies. Mol Biol Cell. 2002;13:723–737. doi: 10.1091/mbc.01-03-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bozon V, Di Scala E, Eftekhari P, Hoebeke J, Lezoualc'h F, Fischmeister R, Argibay J. Agonist-like activity of antibodies directed against the second extracellular loop of the human cardiac serotonin 5-HT 4(e) receptor in transfected COS-7 cells. Receptors Channels. 2002;8:113–121. [PubMed] [Google Scholar]
- 51.Peter JC, Eftekhari P, Billiald P, Wallukat G, Hoebeke J. scFv Single chain antibody variable fragment as inverse agonist of the β2-adrenergic receptor. J Biol Chem. 2003;278:36740–36747. doi: 10.1074/jbc.M306877200. [DOI] [PubMed] [Google Scholar]
- 52.Takao M, Iwasa T, Yamamoto H, Takeuchi T, Tokunaga F. Anti-bovine rhodopsin monoclonal antibody recognizing light-dependent structural change. Zoolog Sci. 2002;19:651–659. doi: 10.2108/zsj.19.651. [DOI] [PubMed] [Google Scholar]
- 53.Gupta A, Décaillot F, Gomes I, Tkalych O, Heimann AS, Ferro E, Devi LA. Conformation State-sensitive Antibodies to G-protein-coupled Receptors. J Biol Chem. 2007;282:5116–5124. doi: 10.1074/jbc.M609254200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gupta A, Rozenfeld R, Gomes I, Raehal KM, Décaillot FM, Bohn LM, Devi LA. Post-activation-mediated changes in opioid receptors detected by N-terminal antibodies. J Biol Chem. 2008;283:10735–44. doi: 10.1074/jbc.M709454200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heimann AS, Gomes I, Dale CS, Pagano RL, Gupta A, de Souza LL, Luchessi AD, Castro LM, Giorgi R, Rioli V, Ferro ES, Devi LA. Hemopressin is an inverse agonist of CB1 cannabinoid receptors. Proc Natl Acad Sci USA. 2007;104:20588–20593. doi: 10.1073/pnas.0706980105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gupta A, Heimann AS, Gomes I, Devi LA. Antibodies against G-protein coupled receptors: novel uses in screening and drug development. Combinatorial Chemistry & High Throughput Screening. 2008;11:463–467. doi: 10.2174/138620708784911465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dragun D, Philippe A, Catar R, Hegner B. Autoimmune mediated G-protein receptor activation in cardiovascular and renal pathologies. Thromb Haemost. 2009;101(4):643–648. [PubMed] [Google Scholar]
- 58.Gomes I, Gupta A, Singh SP, Sharma SK. Monoclonal antibody to the delta opioid receptor acts as an agonist in dual regulation of adenylate cyclase in NG108-15 cells. FEBS Lett. 1999;456:126–130. doi: 10.1016/s0014-5793(99)00878-9. [DOI] [PubMed] [Google Scholar]
- 59.Mijares A, Lebesgue D, Wallukat G, Hoebeke J. From agonist to antagonist: Fab fragments of an agonist-like monoclonal anti-beta 2-adrenoceptor antibody behave as antagonists. Mol Pharmacol. 2000;58:373–379. doi: 10.1124/mol.58.2.373. [DOI] [PubMed] [Google Scholar]
- 60.Deng HB, Yu Y, Pak Y, O'Dowd BF, George SR, Surratt CK, Uhl GR, Wang JB. Role for the C-terminus in agonist-induced μ opioid receptor phosphorylation and desensitization. Biochem. 2000;39(18):5492–5499. doi: 10.1021/bi991938b. [DOI] [PubMed] [Google Scholar]
- 61.Almagro JC, Fransson J. Humanization of antibodies. Front Biosci. 2008;13:1619–1633. doi: 10.2741/2786. [DOI] [PubMed] [Google Scholar]
- 62.Carter P. Potent antibody therapeutics by design. Nature Reviews Immunology. 2006;6:343–357. doi: 10.1038/nri1837. [DOI] [PubMed] [Google Scholar]