Figure 4.
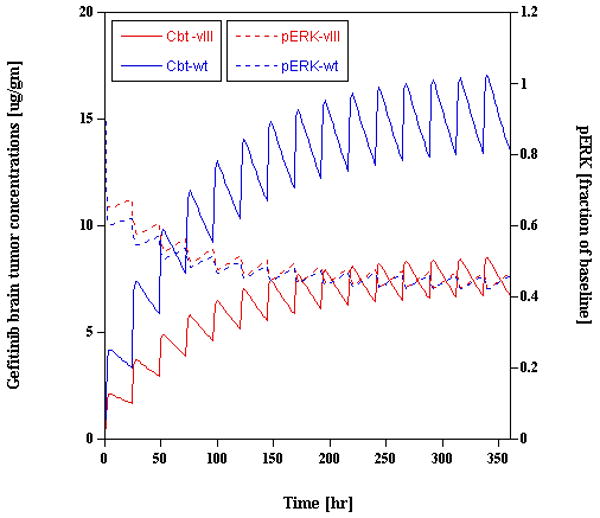
Physiologically-based pharmacokinetic/pharmacodynamic model-predicted gefitinib brain tumor concentrations [left y-axis] and fractional inhibition of pERK [right y-axis] in patients with either wild-type EGFR or mutant vIIIEGFR tumors. To obtain an analogous pERK profile in both tumor types, a regimen of 1000 mg/d or 500 mg/d of gefitinib is predicted in the wild-type EGFR and vIIIEGFR groups, respectively. The simulation used a 15-day duration of gefitinib administration (adapted from reference 13).
