Abstract
Myocardial oxygen extraction fraction (OEF) during hyperemia can be estimated using a double-inversion-recovery (DIR) prepared T2-weighted black-blood sequence. Severe irregular ECG-triggering due to elevated heart rate and/or arrhythmias may render it difficult to adequately suppress the flowing left ventricle blood signal and thus potentially cause errors in the estimates of myocardial OEF. Thus, the goal of this study was to evaluate another black-blood technique, a diffusion-weighted (DW)-prepared TSE sequence for its ability to determine regional myocardial OEF during hyperemia. Control dogs and dogs with acute coronary artery stenosis were imaged with both the DIR- and DW-prepared TSE sequences at rest and during either dipyridamole or dobutamine hyperemia. Validation of MRI OEF estimates was performed using blood sampling from the artery and coronary sinus in control dogs. The two methods showed comparable correlations with blood sampling results (R2 = 0.9). Similar OEF estimations for all dogs were observed except for the group of dogs with severe coronary stenosis during dobutamine stress. In these dogs, the DW method provided more physiologically reasonable OEF (hyperemic OEF = 0.75 ± 0.08 vs resting OEF of 0.6) than the DIR method (hyperemic OEF = 0.56 ± 0.10). DW-preparation may be a valuable alternative for more accurate oxygenation measurements during irregular ECG-triggering.
Keywords: MRI, myocardium, BOLD, oxygen extraction
INTRODUCTION
Coronary artery disease results in an imbalance between myocardial oxygen supply and demand. The balance of these two physiological indices is reflected in the myocardial oxygen extraction fraction (OEF), defined as ([O2]artery−[O2]vein)/[O2]artery. As OEF reflects the combined effects of blood flow and oxygen consumption, evaluating OEF could help assess myocardial ischemia. We have recently shown that changes in myocardial T2 values obtained with the blood oxygen level-dependent (BOLD) effect are related to the myocardial OEF during hyperemia in normal dogs (1, 2, 3) as well as moderately stenotic dogs (4, 5). In these previous studies, a double-inversion recovery (DIR)-prepared turbo spin echo (TSE) sequence was utilized to obtain black-blood T2-weighted images (6), and to determine the myocardial OEF during dipyridamole or dobutamine induced hyperemia. Dipyridamole is a potent vasodilator, which increases oxygen supply without significantly affecting oxygen demand. Dobutamine is a β-adrenergic agonist that results in increased contractility and cardiac output. To support this increase in demand, normal myocardium experiences an increase in oxygen supply. Therefore, myocardial oxygen supply and demand remains coupled in normal myocardium during dobutamine stress, and is decoupled during dipyridamole vasodilation.
Dobutamine has been widely used for the diagnosis of ischemia in cardiac stress testing. However, increased heart rate (HR) and/or arrhythmias during dobutamine stress (7) can result in erroneous ECG-triggering which may significantly reduce the DIR-TSE sequence’s ability to suppress left ventricle blood signals. The severe coronary artery stenosis further exaggerates this problem. Diffusion-weighting prepared sequences have been used for black-blood imaging in the liver (8), carotid artery (9), and heart (10). Therefore, the purpose of this study was to evaluate a diffusion-weighted (DW)-prepared TSE sequence for its ability to determine regional myocardial OEF during dipyridamole or dobutamine induced hyperemia. This pilot study was performed in control dogs as well as dogs with acute coronary stenosis.
MATERIALS AND METHODS
Animal Procedures
All animal procedures were approved by the animal studies committee of our institution. 17 mongrel dogs (weight: 20.9 ± 6.9 kg) were divided into 3 groups based on luminal area stenosis: 0% stenosis controls (n = 7), 50% stenosis (n = 3) and 70-90% stenosis (n = 7) (Table 1), which represents normal, mild, and moderate stenosis, respectively. Studies typically lasted 4-5 hours, with 1.5 hours for stenosis surgery. Anesthesia was induced with 12.5 mg/kg sodium thiopental. Dogs were then intubated and ventilated with medical air at a tidal volume of 12 ml/kg and a rate of 10-15 breaths/minute. Anesthesia was maintained with ventilated 1-2% Isoflurane. Bilateral femoral cut-downs were then performed. A femoral artery catheter was connected to a fluid filled transducer for invasive blood pressure monitoring. Another catheter was placed into a femoral vein for the administration of fluids as well as dipyridamole or dobutamine. For the dogs given stenosis, a thoracotomy was performed in the left 4th intercostal space. Proximal left-anterior descending (LAD) stenosis was created with a homemade, screw-type MR-compatible stenosis clamp. Stenosis severity was confirmed by Doppler coronary hyperemic flow reduction as previously described (11). Briefly, several 20-sec occlusions were performed to determine the reactive hyperemic flow response. After tightening the stenosis clamp, another 20-sec occlusion was performed to determine the reduction in reactive hyperemic flow response. Once the desired stenosis severity was set, defined by the reduction in reactive hyperemic flow response (11), the occluder and Doppler flow probe were removed, leaving only the MR-compatible stenosis clamp. The chest remained open, and care was taken to assure stenosis position remained constant during animal positioning into the MR scanner.
Table 1.
Division of dog groups and MR-derived hyperemic OEF data (rest OEF assumed = 0.6).
| Group (n) | Hyperemia | LAD Stenosis | DIR OEF | DW OEF | ||
|---|---|---|---|---|---|---|
| Global | Global | |||||
| Control Dogs | ||||||
| Group 1 (7) | DIP | 0% | 0.29 ± 0.11 | 0.32 ± 0.09 | ||
| DIR OEF | DW OEF | |||||
| LAD | LCx | LAD | LCx | |||
| LAD Stenotic Dogs | ||||||
| Group 2 (3) | DOB | 50% | 0.60 ± 0.06 | 0.66 ± 0.12 | 0.61 ± 0.02 | 0.68 ± 0.15 |
| Group 3 (7) | DOB | 70-90% | 0.56 ± 0.10 | 0.52 ± 0.13 | 0.75 ± 0.08†* | 0.60 ± 0.11 |
DIP, dipyridamole; DOB, dobutamine; LAD, left anterior descending; LCx, left circumflex.
P < 0.05 between LAD and LCx or between Group 2 and Group 3.
P < 0.01 between DIR and DW methods.
To validate the MR OEF measurements using the DW method, the seven control dogs underwent arterial and venous (AV) blood sampling. To create a large range of OEF values, dipyridamole was injected in these seven dogs. One catheter was inserted from the femoral artery into the arterial system, while a second catheter entered from the right external jugular vein into the coronary sinus. The placement of the coronary sinus catheter was performed under fluoroscopic guidance as previously described (12). Blood sampling was not performed in dogs with stenosis because regional differences in hyperemic OEF caused by the stenosis cannot be measured by AV sampling. The AV samples were analyzed with a hemoximeter (Stat Profile pHOx Plus C, Nova Biomedical Corporation, Waltham, MA), which measured both %HbO2 and hematocrit. HR and blood pressure were monitored continuously using a MR-compatible hemodynamic monitor (Vital Signs Monitoring System, Invivo Research, Orlando, FL). After surgery, the dogs with stenosis remained open-chest and were moved to the MR imaging suite. Control dogs did not undergo the thoracotomy surgery. Because dobutamine often induces severe cardiac motion artifacts, in all stenotic dogs, dobutamine was infused to evaluate the performance of the DW method
For imaging during hyperemia, dipyridamole was injected intravenously at a dose of 0.14 mg/kg/min for 4 min or dobutamine was titrated intravenously at 10 μg/kg/min increments every 5 min up to a maximum of 30 μg/kg/min, or until the heart rate was greater than 150 bpm.
MR Imaging
All dogs were imaged with a whole-body 1.5T Sonata scanner (Siemens Medical Solutions, Erlanger, Germany). A four-element phased array coil placed around the dogs’ chest was used for signal reception and a body coil was used as a transmitter. Control dogs were positioned supine, while dogs with stenosis were positioned on the right lateral position to preserve the stenosis placement. Scout imaging was performed to obtain a short-axis image of the left ventricle (LV) at the mid-cavity level. Cine imaging was performed to determine the motionless period of the cardiac cycle. During all scans, respiratory motion was reduced by turning off the ventilator (30 s max).
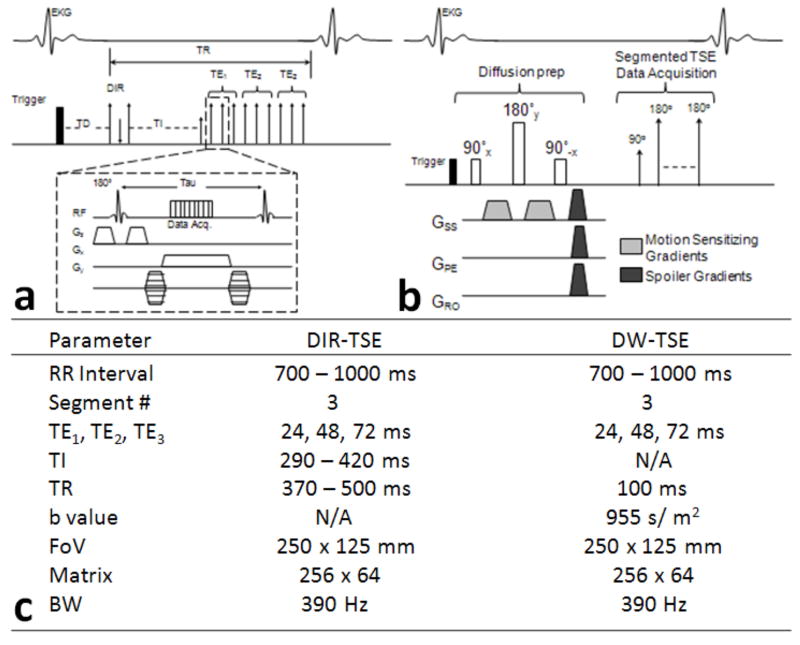
BOLD imaging was performed with both the DIR- and DW-prepared TSE methods in all dogs at rest and during either dipyridamole or dobutamine hyperemia. DIR and DW sequence diagrams and parameters are found in Figure 1. For both the DIR- and DW-prepared methods, the segmented Carr-Purcell-Meiboom-Gill (CPMG) TSE sequence was structured with three segmentations of three images acquired at different TEs. In other words, three images were acquired per breath hold, one at each of three TEs, using a segmented k-space acquisition. T2-weighted images were acquired at two echo spacing times (τ), with both τ1=8 and τ2=12 ms and the same TEs of 24, 48, 72 ms at BW = 400 Hz/pixel. During hyperemia, only T2-weighted images with τ1 of 8 ms were collected. The segmented k-space data for all three T2-weighted images was collected in the same cardiac cycle at mid-diastole when cardiac contractile motion was minimal. For both BOLD sequences, the segment # used was three (turbo factor = 3), and a total of 66-84 k-space lines were collected in 22-28 cardiac cycles. For the DW-prepared pulses, only gradients along the slice direction were used for the diffusion weighting with a duration of 4 ms and a magnitude of 4 mT/m, resulting in a b value of 1695 sec/m2 or 0.0.0017 sec/mm2. The diffusion effect was almost negligible. These diffusion parameters were selected based on acceptable image quality by adjusting these parameters in the initial control dog studies. For both BOLD sequences, typically 2 to 3 measurements were performed at each condition (rest and dipyridamole or dobutamine), and the T2 results were averaged. The inversion time (TI) used for the DIR-TSE sequence depended on the heart rate, or RR interval, and were derived based on a previously reported method (13).
FIG. 1.
Sequence diagrams for the double-inversion recovery (DIR)-prepared TSE (a) and the diffusion weighted (DW)-prepared TSE (b). Comparison of sequence parameters (c). GSS, slice-encoding gradient; GPE, phase-encoding gradient; GRO, read out gradient.
To evaluate the accuracy of T2 measurements using DIR or DW methods, a newly developed T2prep sequence was used in 4 dogs in group 3 as T2 reference measurements. The sequence is similar to the T2prep-TrueFISP reported previously (14), except that readout is gradient echo acquisition with TR/TE of 3.2/1.8 msec and a flip angle of 15°. Five echoes can be acquired within one breath-hold with optimized TEs of 24, 36, 48, 60, and 72 msec. Other imaging parameters were the same as those of DIR sequence.
The OEF quantification model, described below, requires the measurement of MBV both at rest and during hyperemia (2). These values were obtained with first-pass perfusion MRI (15). Images during the 15 μmol/kg bolus injection of an intravascular contrast agent, Gadomer (Bayer Schering Pharma AG, Berlin, Germany), were acquired by a saturation prepared turbo fast low-angle shot (SR-FLASH) sequence at a resolution of 2.1 mm × 0.9 mm and a slice thickness of 8 mm. One short-axis slice of the LV was acquired during each scan at the same location as the BOLD imaging. There were 60-80 dynamic images collected at a rate of one image per RR interval. Other imaging parameters included: TR = 2.5 ms; TE = 1.2 ms; TI = 90 ms; flip angle = 18°; FoV = 220 mm × 138 mm; matrix size = 128 × 80%; BW = 675 Hz/pixel; and image acquisition time window per cardiac cycle = 150 ms. The entire study, from the preparation of animals to the end of MRI scans, last approximately 4 hours.
Data Analysis
BOLD images were analyzed and T2 and OEF maps were created using homemade software (Matlab 6.5.1, The MathWorks, Inc., Natick, MA). Regional myocardial T2 values were estimated by drawing transmural regions of interest (ROIs) on both the LAD-perfused region and the remote left circumflex (LCx)-perfused region. These ROIs encompassed 150-200 pixels on average. One independent observer, unknown to the stenotic degree, drew the ROIs on both DIR and DW images to calculate OEF. Another independent observer drew ROIs on DW images for the measurements of OEF to assess inter-observer correlation. These ROIs were not identical between DIR and DW images; however care was taken to assure similar shape and location. ROI placement in the anterior (LAD-perfused region) was determined by concurrently looking at the first-pass perfusion images which easily demarcate the region of perfusion deficit. Using a two-compartment model, the hyperemic OEF can be determined (2, 3, 4). This previously described relationship that allows us to determine the hyperemic OEF from the myocardial T2 measurement is defined as:
| [1] |
| [2] |
where blood T2 or T2b is calculated using the model reported by van Zijl and Oja et al (16,17), T2t is the measured myocardial T2 during hyperemia, R20t is the reciprocal of the T2 of the myocardial tissue, R21t is the reciprocal of the T2 of the extravascular space, and τ is the inter-echo spacing time. Therefore, by varying the inter-echo spacing time τ, measurements of apparent myocardial T2 at rest can determine the parameters R20t and R21t for each subject with a known OEF and MBV at rest. Then during hyperemia, only one inter-echo spacing time is needed (τ = 8 ms) to determine the change in T2 with hyperemia. Rest OEF was assumed to be 0.6, which is based on OEF values measured in control dogs by the arterial and coronary sinus blood sampling approach (2). It is assumed that this value changes little with moderate stenosis, as shown by positron emission tomography (PET) (18).
Dynamic first-pass perfusion images were analyzed by another homemade software system (Java Runtime Environment V5.0, Sun Microsystems, Santa Clara, CA) developed in our laboratory. Images were first temporally and spatially denoised (19), and then subjected to a validated perfusion quantification algorithm (15, 20), similar to the established B-Spline method (21). ROIs were then drawn on the created MBV maps in both the LAD-perfused and remote LCx-subtended areas for regional comparison.
Image quality was determined by signal-to-noise ratio (SNR) and uniformity. The SNR was determined from a global LV myocardial ROI in control dog images, or a ROI placed in the remote (normal) regions of the dogs subjected to LAD stenosis. Noise was recorded from the standard deviation of an ROI drawn in the background air of the image, corrected for the use of multiple phased array coils (22). Image uniformity was determined by using the same LV myocardial ROIs as for the SNR, and dividing the myocardial signal standard deviation by the intensity value. These measurements were recorded for all three TEs of the T2-weighted BOLD images.
All data were averaged for the groups, and presented as mean ± SD. Statistical analysis was performed with a paired t-test to compare between the DIR- and DW-preparation methods, as well as between the MRI and AV sampling OEF data. Linear regression was performed to compare the OEF data obtained with the different methods. P < 0.05 was considered statistically significant.
RESULTS
Hemodynamic Results
Hemodynamic data is found in Table 2. Overall, dipyridamole infusion resulted in a non-significant increase in HR and rate pressure product (RPP); while dobutamine hyperemia caused significant (P < 0.05) increases in both HR and RPP.
Table 2.
Dog hemodynamics at rest and during dipyridamole or dobutamine hyperemia.
| Control Dogs | LAD Stenosis Dogs | ||||||
|---|---|---|---|---|---|---|---|
| HR | BP | RPP / 100 | %HbO2 | HR | BP | RPP / 100 | |
| Rest | 82 ± 23 | 108 ± 27 | 83 ± 12 | 38 ± 15 | 110 ± 12 | 93 ± 11 | 106 ± 15 |
| DIP | 89 ± 12 | 99 ± 18* | 87 ± 13 | 83 ± 11 | 98 ± 28 | 101 ± 10* | 118 ± 20 |
| DOB | 149 ± 0.0 | 108 ± 0.0 | 161 ± 0.0 | 31 ± 0.0 | 137 ± 24* | 110 ± 6* | 150 ± 20* |
%HbO2 collected from the coronary sinus in control dogs only. DIP, dipyridamole; DOB, dobutamine; HR, heart rate; RPP, rate pressure product; SBP, systolic blood pressure.
P < 0.05 compared to rest.
T2 - OEF Results
The average T2 ± SD values for each group are shown in Table 3. Overall, DW-derived T2 values are ~6% higher, than the DIR-determined T2 values for control dogs (P = NS). In dogs with LAD stenosis, this difference disappears in LAD regions, but LCx T2 was slightly higher (~3%) with the DW method in group 3 dogs. In contrast, in stenotic dogs of group 3, LAD T2 values obtained with the DIR method was actually lower, suggesting that flow artifacts in DIR images may be the dominant error source.
Table 3.
Average T2 data for each group for the DIR and DW BOLD methods.
| Group | DIR |
DW |
|||
|---|---|---|---|---|---|
| Global T2 | Global T2 | ||||
| 1 | Rest | 46.0 ± 1.5 | 50.8 ± 3.6 | ||
| DIP | 52.9 ± 2.5* | 56.2 ± 3.6* | |||
| DIR |
DW |
||||
| LAD T2 |
LCx T2 |
LAD T2 |
LCx T2 |
||
| 2 | Rest | 52.3 ± 0.5 | 52.2 ± 0.6 | 51.6 ± 1.0 | 51.7 ± 0.2 |
| DOB | 52.0 ± 0.4 | 52.6 ± 0.3 | 51.3 ± 1.2 | 52.2 ± 0.6 | |
| 3 | Rest | 52.8 ± 3.2 | 51.9 ± 2.7 | 52.6 ± 2.8 | 53.5 ± 2.3† |
| DOB | 52.2 ± 3.5 | 51.6 ± 2.8 | 50.7 ± 3.7† | 52.9 ± 2.8 | |
DIP, dipyridamole; DOB, dobutamine; LAD, left anterior descending; LCx, left circumflex.
P < 0.05 between rest and stress.
P < 0.05 between DIR and DW methods.
Bland-Altman plots in Figure 2 (a) and (b) demonstrated the comparison between T2prep and DIR or DW measurements for myocardial T2 in 4 stenotic dogs, using T2 data in LAD and LCx regions. The mean errors were 1.9% (95% CI, -7.1 – 10.9%) and -1% (95% CI, -9.8 – 7.8%) for DIR and DW methods, respectively, indicating no systematic errors in both measurements. There was an excellent correlation between observers for the measurement of myocardial OEF (Figure 2 (c)). The slope was 1.01 and the mean OEF difference was merely -0.01 ± 0.075. The average OEF ± SD values for each group are shown in Table 1. Both MR techniques agreed strongly with the AV blood sampling results (Figure 3). On average, the OEF obtained by the DIR method appeared to slightly underestimate the OEF, but this difference is not significant. As expected, dipyridamole vasodilation caused significant decreases in OEF (P < 0.05), due to large increases in O2 supply without increases in O2 demand. On the other hand, dobutamine caused no significant changes in OEF because of the balance between the increases in myocardial contractility (O2 demand) and O2 supply. Image quality appeared comparable in normal dogs; representative images are shown in Figure 4a.
FIG. 2.
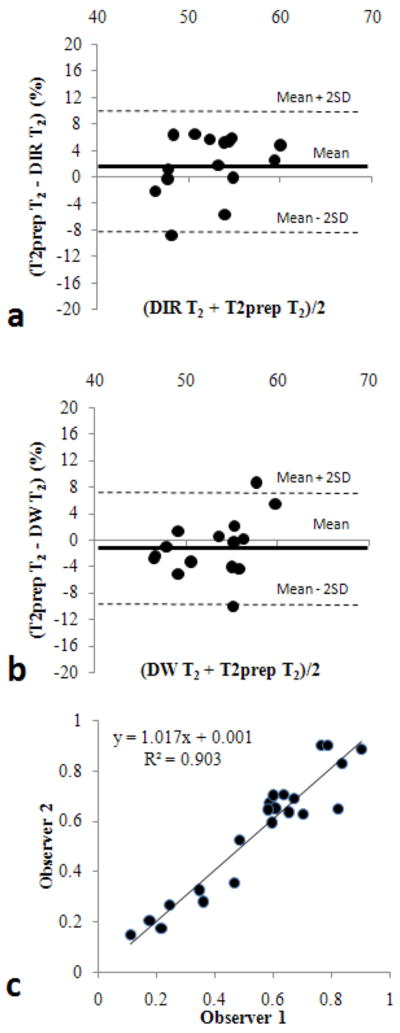
Bland-Altman plots for the comparison between T2prep and DIR measurements (a), and between T2prep and DW measurements (b). Interobserver variability in T2 measurements using DW method in all dogs is shown in (c).
FIG. 3.
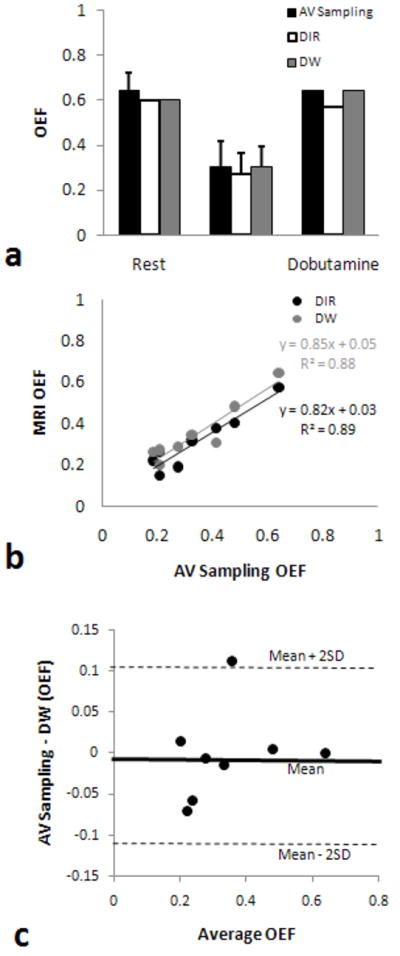
AV sampling and MRI OEF comparisons of the control dogs. Group average values show a slight underestimation by the DIR prepared method (a). Regression analysis between the AV sampling and MR OEF shows strong correlation between both MR methods (b). Bland-Altman plot (c) to compare DIR with DW methods for the measurements of regional myocardial OEF.
FIG. 4.
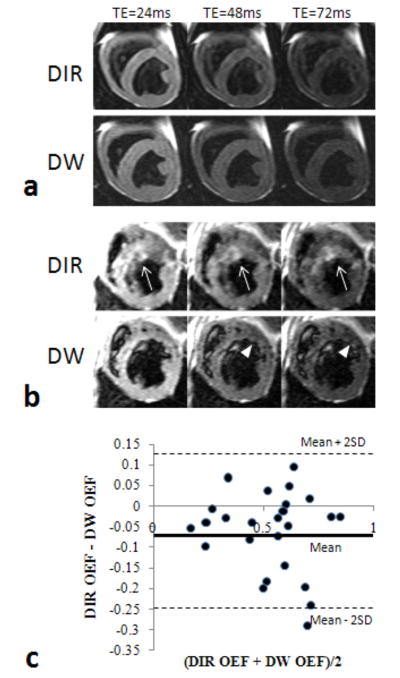
Sample T2-weighted images, containing the three TEs acquired with the DIR and DW methods. Images from a control dog during rest (a), and from a dog with an LAD stenosis during dobutamine stress (b). DIR images often contained poor blood saturation, often resulting in “ghosting” artifacts that affect the perceived LV wall border (arrows). DW images show better blood saturation, allowing the perfusion defects caused by a stenosis to be visualized (arrowheads).
Myocardial blood flow reserve, determined from first-pass perfusion imaging, show progressively reduced values in LAD regions from group 2 (50% stenosis, 1.6 ± 0.1) to group 3 (70-90% stenosis, 1.3 ± 0.2, P < 0.05). Group 2 dogs with mild (50%) LAD stenosis given dobutamine showed no significant changes in OEF from resting condition in either the LAD- or normal LCx-subtended regions, nor the differences between DIR and DW methods. Lastly, the group 3 dogs with moderate (70-90%) LAD stenosis given dobutamine did show a significant difference between the DIR- and DW-prepared methods (Table 1). This group of dogs experienced significantly greater ECG-triggering problems due to increased heart rate and arrhythmia. The DIR technique showed a slight decrease in OEF in the remote inferior region, while the stenotic anterior region showed no significant change in OEF from the resting condition. The DW technique, however, showed a significant increase in OEF in the stenosis subtended region, and no significant change in OEF in the remote inferior region. The DW-derived OEF values indicate that during dobutamine a severe coronary stenosis could limit the O2 supply during hyperemia, and the OEF increases to match myocardial contractile demand. The DIR images during dobutamine showed flow artifacts in group 3 dogs, particularly on the anterior region (Figure 4b). These may have lead to overestimation of myocardial T2 and thus lower OEF. Figure 4c shows Bland-Altman analysis between DIR OEF and DW OEF from pooled data of all dogs. Clearly, the DIR OEF underestimated DW OEF (mean = -0.06, 95% CI, -0.25 to 0.13), mainly due to the lower OEF estimated in group 3 dogs. It should be noted that there were no dogs in group 3 with DIR images completely void of artifacts, so in no case did the DIR OEF match the DW OEF for this group.
The SNR between DIR and DW images was not statistically different (Figure 5a). The image uniformity results between DIR and DW images were also not statistically different (Figure 5b). As expected, the uniformity is increased with longer echo times as SNR decreases. All differences between TEs for both SNR and uniformity were statistically significant (P < 0.05).
FIG. 5.
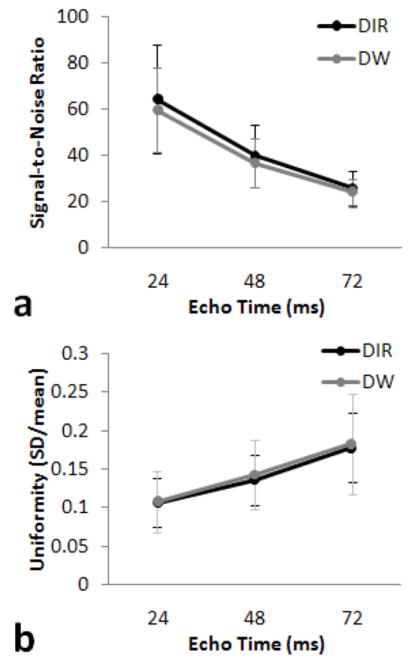
Average signal to noise ratio (SNR) versus echo time (a). Average uniformity versus echo time (b).
DISCUSSION
In this report, a diffusion-weighted TSE sequence was developed for black-blood TSE imaging to estimate myocardial OEF during hyperemia. Due to a very low b value, SNR was preserved with a similar imaging quality to DIR images used previously (2). Control dogs and dogs with moderate stenosis during dobutamine hyperemia show no significant differences in OEF between the two MR methods, as validated by the invasive AV blood sampling method. Mild stenosis did not cause a significant increase in OEF, indicating autoregulation may remain effective for non-critical stenosis. An earlier study also showed only slight increases in OEF during dobutamine hyperemia with moderate stenosis, and was confirmed by PET measurements (5). The DW-TSE approach demonstrates better saturation of LV blood signals when severe irregular ECG triggering occurs during dobutamine. In the more severe stenosis subtended region, myocardial blood flow could not be able to meet oxygen demand so OEF would have to increase to prevent ischemia. Hyperemic OEF increased significantly in this region compared to LCx region or LAD regions subtended by moderate stenosis (Group 2), as observed by DW-TSE imaging, while the OEF in the same region failed to change, as measured by the DIR-TSE imaging method.
It is thought that this DIR OEF underestimation is a result of inadequate ECG-triggering, resulting in inefficient saturation of LV blood signals using double inversion pulses. The rapid change in heart rate from dobutamine and occasional arrhythmias caused by severe stenosis compounded these errors; often leading to cardiac motion and incomplete blood saturation on DIR-prepared images. The DW prepared method is less susceptible to these effects, and images showed fewer flow artifacts around the blood-myocardial boundary (Figure 4). To semi-quantify these results, the heterogeneity of the LV bloodpool was determined by measuring the standard deviation of the LV blood signal. It shows that the group 2 dogs with mild stenosis have an average heterogeneity of 26.6 ± 4.0 and 25.9 ± 5.5 for the DIR and DW methods, respectively. However, the group 3 dogs with severe stenosis have heterogeneities of 38.8 ± 12.6 and 30.2 ± 8.3 for the DIR and DW methods, respectively; which are significantly different (P < 0.001). Theoretically, the DW method can replace DIR for our quantification purposes. However, field inhomogeneities may arise in some cases and cause loss of signal at the boundary of lateral myocardium and lung air. In our experience, it is more practical to use the DW method when the DIR method fails to produce acceptable image quality, either at rest or during pharmacologic stress.
We used a much smaller motion-sensitizing gradient for the dephasing effect, compared to the reported DW sequence for black-blood myocardial imaging (10). The main difference was the use of TSE data acquisition in our sequence, which already has an intrinsic black-blood contrast. As described by the report, the flow suppressing effect is mainly from intravoxel phase dispersion of moving spins. The negligible b value and the well suppressed LV flow signals in our study further proved this mechanism.
The accuracy and precision of myocardial OEF measurements depend on the measurement of myocardial T2 and MBV. Our phantom study has indicated < 5% error in the T2 estimation (data not shown) and in vivo validation study suggested an error of < 7.2% in the estimation of MBV (13). Through simple simulations (unpublished), a 10% error in the estimation of MBV resulted in only a 1.5% error for the calculated OEF. The combined errors from both T2 and MBV are approximately < 19% in the OEF estimation. Because in vivo flow artifacts and various motion effects, we expected this accuracy would be larger for the OEF measurement in vivo. Nevertheless, our preliminary finding in 6 subjects using PET as a gold standard in vivo found smaller error of 1.1% in the accuracy and 18-20% precision in the estimation of OEF (5). The precision magnitude agreed with our previously estimated variability of OEF measurement (21%) (3). Further rigorous study in a much larger sample using either DW or DIR preparation is warranted to determine the accuracy and precision of this in vivo OEF measurement. To detect smaller OEF changes, the accuracy of T2 measurements needs substantial improvement. By simulation, T2 accuracy needs to be approximately 2% to achieve a detection limit of 10% in OEF. Other methods to improve the accuracy of myocardial T2 measurement are under investigation in our laboratory.
This study did have several limitations. First of all, there is yet no MRI quantification method for absolute OEF at rest. Thus, we must examine the change in T2 during hyperemia and assume a rest OEF of 0.6, which was validated in control dogs (2). For severe coronary artery disease, this resting OEF may be a systematic error in the estimation of hyperemic OEF. Ongoing efforts are being made to estimate OEF at rest. Regarding with hyperemic OEF, this quantification method assumes that intrinsic myocardial T2 would not change during the imaging session, other than alternations of T2 by pharmacologically induced hyperemia. In the cases of severe stenosis or even infarction, this method may not work precisely due to dynamic changes in myocardial intrinsic T2. Another limitation is that our dogs with LAD stenosis did not have OEF validated by AV blood sampling. This is because AV sampling returns a global OEF result, and therefore would not in any way validate our regional MR-derived OEF results. In vivo PET imaging could be used as a validation approach and is currently investigated in our laboratory. Furthermore, the DW method was only evaluated in stenotic dogs with LAD stenosis for a feasibility study. Stenosis in other coronary arteries, such as right coronary and left circumflex arteries may induce different motion effects. It is not clear how the performance of the DW method would be for these stenoses and such task should be further research objective. A fourth limitation is that only similar (not identical) ROIs were drawn between the DIR, DW, and MBV map images. Care was taken to create similar size ROIs in similar locations; however, this could be a source of error. The fifth limitation is that printed records of the ECG-triggering problems in all dogs could not be obtained. These problems were noted whenever they occurred, and observational notations of this problem were found mostly in the group 3 dogs with significant stenosis during dobutamine. The sixth limitation is of the usage of relatively larger ROIs in the data analysis to improve SNR. Although pixel-by-pixel mapping of OEF was possible (4), significant improvement in SNR and spatial resolution is needed to estimate correct OEFs of endo- and epi-myocardium in a clinical setting. Another limitation was the increased sensitivity of DW-TSE sequence to the field inhomogeneity. Using the 3D autoshimming capability in our 1.5T scanner, this problem was largely minimized.
In conclusion, the DW-prepared TSE black-blood method appears to perform as well as the DIR technique for the quantification of OEF during dipyridamole vasodilation, and better than the DIR method during dobutamine hyperemia. Dobutamine hyperemia involves an increase in HR, and ECG-gating difficulties can be compounded by induced stenosis which can lead to cardiac arrhythmias. Adding DW-prepared sequences into cardiac MR protocols may provide more quality black-blood images, and thus better OEF quantification during times when these severe ECG-triggering irregularities occur.
Acknowledgments
This study was supported by a grant from the National Institutes of Health R01 HL74019.
References
- 1.Balaban RS, Taylor JF, Turner R. Effect of cardiac flow on gradient recalled echo images of the canine heart. NMR Biomed. 1994;7:89–95. doi: 10.1002/nbm.1940070114. [DOI] [PubMed] [Google Scholar]
- 2.Zheng J, Wang J, Nolte M, Li D, Gropler RJ, Woodard PK. Dynamic estimation of the myocardial oxygen extraction ratio during dipyridamole stress by MRI: a preliminary study in canines. Magn Reson Med. 2004;51:718–726. doi: 10.1002/mrm.20025. [DOI] [PubMed] [Google Scholar]
- 3.Zheng J, Wang J, Rowold FE, Gropler RJ, Woodard PK. Relationship of apparent myocardial T2 and oxygenation: towards quantification of myocardial oxygen extraction fraction. J Magn Reson Imaging. 2004;20:233–241. doi: 10.1002/jmri.20111. [DOI] [PubMed] [Google Scholar]
- 4.Zhang H, Gropler RJ, Li D, Zheng J. Assessment of myocardial oxygen extraction fraction and perfusion reserve with BOLD imaging in a canine model with coronary artery stenosis. J Magn Reson Imaging. 2007;26:72–79. doi: 10.1002/jmri.20964. [DOI] [PubMed] [Google Scholar]
- 5.McCommis KS, Zhang H, Herrero P, Gropler RJ, Zheng J. Feasibility study of myocardial perfusion and oxygenation by noncontrast MRI: comparison with PET study in a canine model. Magn Reson Imaging. 2008;26:11–19. doi: 10.1016/j.mri.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simonetti OP, Finn JP, White RD, Laub G, Henry DA. “Black blood” T2-weighted inversion-recovery MR imaging of the heart. Radiology. 1996;199:49–57. doi: 10.1148/radiology.199.1.8633172. [DOI] [PubMed] [Google Scholar]
- 7.Kuijpers D, Janssen CH, van Dijkman PR, Oudkerk M. Dobutamine stress MRI. Part I. Safety and feasibility of dobutamine cardiovascular magnetic resonance in patients suspected of myocardial ischemia. Eur Radiol. 2004;14:1823–1828. doi: 10.1007/s00330-004-2425-y. [DOI] [PubMed] [Google Scholar]
- 8.Hussain SM, De Becker J, Hop WC, Dwarkasing S, Wielopolski PA. Can a single-shot black-blood T2-weighted spin-echo echo-planar imaging sequence with sensitivity encoding replace the respiratory-triggered turbo spin-echo sequence for the liver? An optimization and feasibility study. J Magn Reson Imaging. 2005;21:219–229. doi: 10.1002/jmri.20269. [DOI] [PubMed] [Google Scholar]
- 9.Koktzoglou I, Li D. Submillimeter isotropic resolution carotid wall MRI with swallowing compensation: imaging results and semiautomated wall morphometry. J Magn Reson Imaging. 2007;25:815–823. doi: 10.1002/jmri.20849. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen TD, de Rochefort L, Spincemaille P, Cham MD, Weinsaft JW, Prince MR, Wang Y. Effective motion-sensitizing magnetization preparation for black blood magnetic resonance imaging of the heart. J Magn Reson Imaging. 2008;28:1092–1100. doi: 10.1002/jmri.21568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nohara R, Abendschein DR, Bergmann SR. Transmural gradients of coronary flow reserve with physiologically and morphometrically defined stenoses in dogs. Am Heart J. 1989;118:1167–1175. doi: 10.1016/0002-8703(89)90005-7. [DOI] [PubMed] [Google Scholar]
- 12.Herrero P, Weinheimer CJ, Dence C, Oellerich WF, Gropler RJ. Quantification of myocardial glucose utilization by PET and 1-carbon-11-glucose. J Nucl Cardiol. 2002;9:5–14. doi: 10.1067/mnc.2001.120635. [DOI] [PubMed] [Google Scholar]
- 13.Simonetti OP, Finn JP, White RD, Laub G, Henry DA. “Black blood” T2-weighted inversion-recovery MR imaging of the heart. Radiology. 1996;199:49–57. doi: 10.1148/radiology.199.1.8633172. [DOI] [PubMed] [Google Scholar]
- 14.Huang TY, Liu YJ, Stemmer A, Poncelet BP. T2 measurement of the human myocardium using a T2-prepared transient-state TrueFISP sequence. Magn Reson Med. 2007;57:960–966. doi: 10.1002/mrm.21208. [DOI] [PubMed] [Google Scholar]
- 15.McCommis KS, Goldstein TA, Zhang H, Misselwitz B, Gropler RJ, Zheng J. Quantification of myocardial blood volume during dipyridamole and dobutamine stress: a perfusion CMR study. J Cardiovasc Magn Reson. 2007;9:785–792. doi: 10.1080/10976640701545206. [DOI] [PubMed] [Google Scholar]
- 16.van Zijl PC, Eleff SM, Ulatowski JA, Oja JM, Ulug AM, Traystman RJ, Kauppinen RA. Quantitative assessment of blood flow, blood volume and blood oxygenation effects in functional magnetic resonance imaging. Nat Med. 1998;4:159–167. doi: 10.1038/nm0298-159. [DOI] [PubMed] [Google Scholar]
- 17.Oja JM, Gillen JS, Kauppinen RA, Kraut M, van Zijl PC. Determination of oxygen extraction ratios by magnetic resonance imaging. J Cereb Blood Flow Metab. 1999;19:1289–1295. doi: 10.1097/00004647-199912000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Buck A, Wolpers HG, Hutchins GD, Savas V, Mangner TJ, Nguyen N, Schwaiger M. Effect of carbon-11-acetate recirculation on estimates of myocardial oxygen consumption by PET. J Nucl Med. 1991;32:1950–1957. [PubMed] [Google Scholar]
- 19.Goldstein TA, Zhang H, Misselwitz B, Gropler RJ, Zheng J. Improvement of quantification of myocardial first-pass perfusion mapping: a temporal and spatial wavelet denoising method. Magn Reson Med. 2006;56:439–445. doi: 10.1002/mrm.20950. [DOI] [PubMed] [Google Scholar]
- 20.Goldstein TA, Jerosch-Herold M, Misselwitz B, Zhang H, Gropler RJ, Zheng J. Fast mapping of myocardial blood flow with MR first-pass perfusion imaging. Magn Reson Med. 2008;59:1394–1400. doi: 10.1002/mrm.21559. [DOI] [PubMed] [Google Scholar]
- 21.Jerosch-Herold M, Swingen C, Sethamraju RT. Myocardial blood flow quantification with MRI by model-independent deconvolution. Med Phys. 2002;29:886–897. doi: 10.1118/1.1473135. [DOI] [PubMed] [Google Scholar]
- 22.Constantinides CD, Atalar E, McVeigh ER. Signal-to-noise measurements in magnitude images from NMR phased arrays. Magn Reson Med. 1997;38:852–857. doi: 10.1002/mrm.1910380524. [DOI] [PMC free article] [PubMed] [Google Scholar]