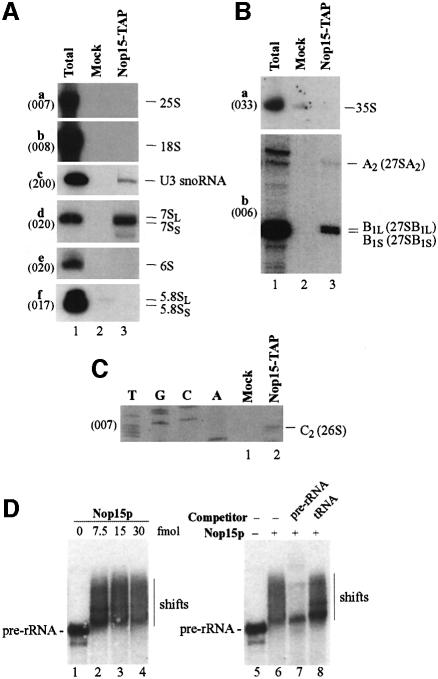
Fig. 4. Nop15p associates with pre-rRNAs in vivo and in vitro. (A–C) Nop15p-TAP co-precipitates with pre-rRNAs. Lane 1, total RNA control (5 µg); lane 2, mock precipitate from a wild-type control strain; lane 3, precipitate from a strain expressing Nop15p-TAP. (A) Northern hybridization of high and low molecular weight RNAs separated on a 1.2% agarose/formaldehyde gel or 8% polyacrylamide/urea gel, respectively. (B and C) Primer extension analyses. Nop15p-TAP was immunoprecipitated from cell lysates using IgG–agarose, with release of bound RNA–protein complexes by cleavage of the protein A linker by TEV protease. RNA was recovered from the released material, and from a mock-treated, isogenic wild-type control strain. Oligonucleotides used are indicated in parentheses. (D) Nop15p binds to pre-rRNA in vitro. Gel mobility shift assay performed with an in vitro transcribed pre-rRNA fragment, extending from the 5′ region of ITS1 to the 3′ region of ITS2. Nop15p was expressed in E.coli as a GST fusion with a thrombin-sensitive linker and eluted from a glutathione–Sepharose column by cleavage with thrombin. Lanes 1–4, 10 fmol pre-rRNA incubated with 0–30 fmol Nop15p as indicated; lanes 5–8, competition experiment. The gel shift assay was performed in the presence of 200-fold molar excess of cold pre-rRNA (lane 7) and tRNA (lane 8). Reactions in lanes 6–8 contained 10 fmol RNA and 15 fmol Nop15p. Complexes were resolved by electrophoresis in native 6% acrylamide/bisacrylamide (80:1) gels containing 0.5× TBE.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.