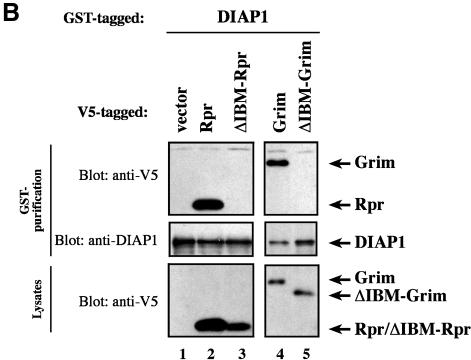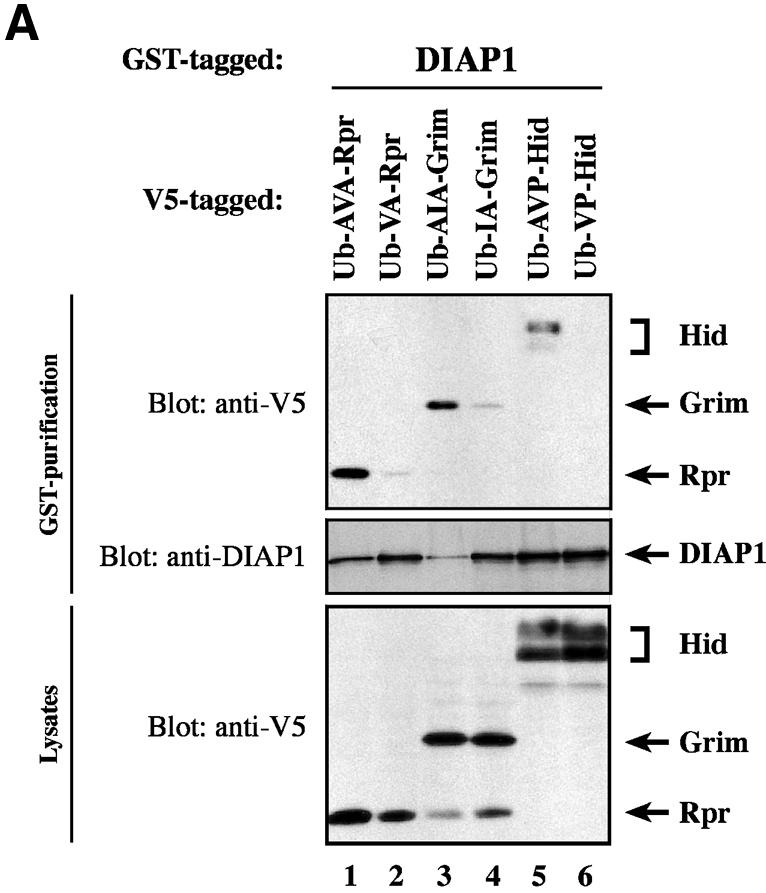
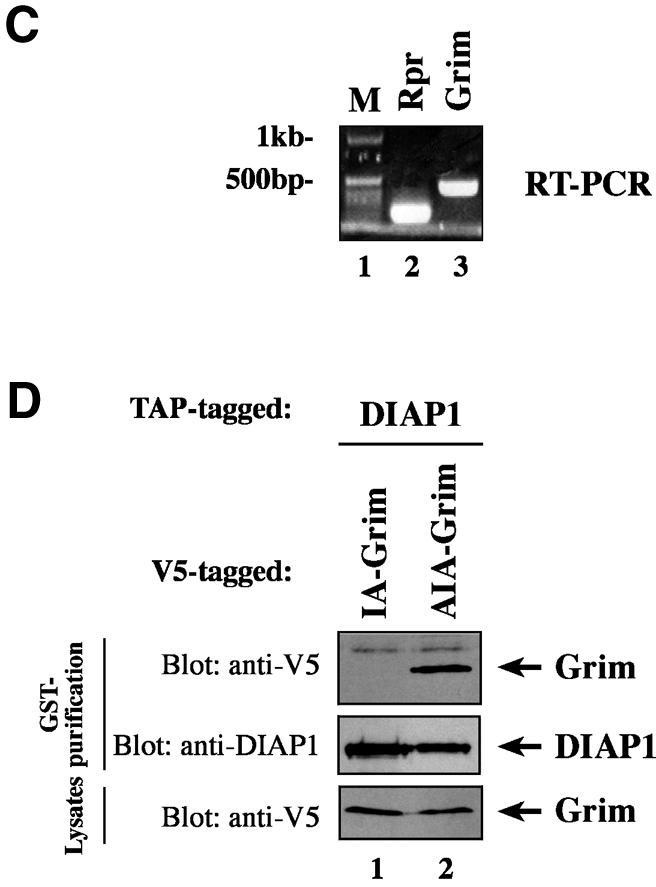
Fig. 6. Ala1 of the IBM of Rpr, Grim and Hid is indispensable for their binding to DIAP1. (A) Co-purification of Rpr-V5, Grim-V5 or Hid-V5 with DIAP1–GST from cellular extracts. Wild-type but not mutant Rpr, Grim or Hid lacking Ala1 efficiently interacted with DIAP1. Rpr, Grim and Hid were expressed using the ubiquitin fusion technique. Protein expression (bottom panel) and co-purification (top panel) was examined by immunoblot analysis with the indicated antibodies. Purification of DIAP1–GST was verified by western blot analysis of the eluate using anti-DIAP1 RING antibody (middle panel). (B) Rpr and Grim proteins that lack their entire IBM completely failed to bind to DIAP1. Note, while the difference in mobility between Grim and ΔIBM-Grim is readily detectable, Rpr and ΔIBM-Rpr appear to migrate at the same level due to the SDS–acrylamide gel used. (C) Detection of rpr and grim mRNAs by RT–PCR from total RNA of healthy S2 cells. (D) No residual binding of IA-Grim to DIAP1 is detectable in 293 cells suggesting that the residual binding shown in (A) may be due to the association of the Ala1-mutants with endogenous Rpr or Grim proteins.