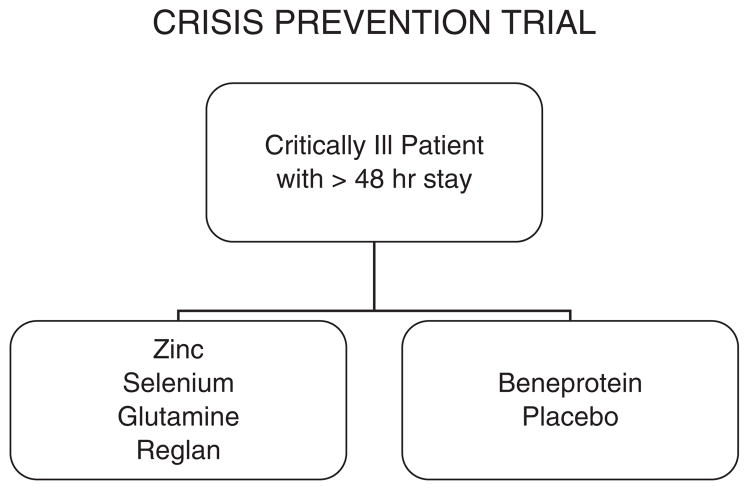
Figure 2.
Patient flow diagram. Patients are randomized in a blind fashion within 48 hours and receive 1 of 2 treatments within 72 hours; either (a) enteral glutamine, zinc, and selenium plus intravenous metoclopramide or (b) enteral Beneprotein™ powder (a whey protein supplement; Nestle Nutrition, Vevey, Switzerland) plus intravenous normal saline. Treatment is continued for whichever occurs first, discharge from the pediatric intensive care unit, loss of enteral tubes and intravenous access, or 28 days.