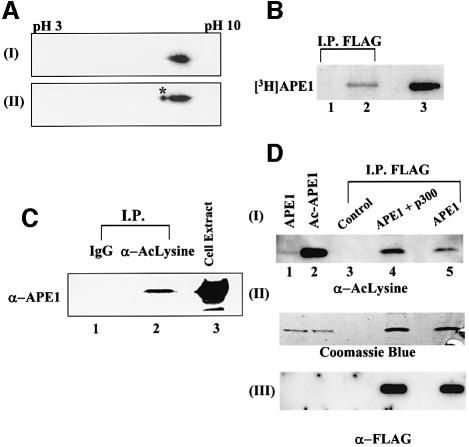
Fig. 1. In vivo acetylation of APE1. (A) Two-dimensional gel electrophoresis of (I) recombinant APE1 (100 ng) and (II) HeLa nuclear extract (50 µg). After isoelectric focusing on an Immobiline dry strip (pH gradient 3–10) in an IPGphor electrophoresis system (Amersham), the proteins were separated by SDS–PAGE, and APE1 was visualized by western blotting. The asterisk indicates modified APE1. (B) Extracts of HCT116 cells transfected with FLAG-tagged APE1 expression plasmid (lane 2) or empty vector (lane 1), after labeling with [3H]Na- acetate, were immunoprecipated with FLAG antibody and analyzed by SDS–PAGE and fluorography. Lane 3: in vitro acetylated [3H]APE1 marker. (C) Western analysis with APE1 antibody of extracts of HCT116 immunoprecepitated with either AcLys antibody (α-AcLys; lane 2) or pre-immune control IgG (lane 1); total cell extract (lane 3) was used as a reference. (D) Panel I: western analysis with AcLys antibody of extracts of HCT116 cells transfected with FLAG-APE1 (lane 5), FLAG-APE1 plus p300 expression plasmids (lane 4), or empty vector (lane 3) followed by immunoprecipitation with FLAG antibody. The specificity of AcLys antibody for AcAPE1 was shown by western analysis of 0.1 µg of APE (lane 1) or AcAPE1 (lane 2). Western analysis of the same blot with FLAG antibody (panel III) and Coomassie staining (panel II) of duplicate immunoprecipitate in the same experiment to show comparable amounts of APE1 in samples with different levels of AcAPE1.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.