Abstract
Precise spatiotemporal control of Gata1 expression is required in both early hematopoietic progenitors to determine erythroid/megakaryocyte versus granulocyte/monocyte lineage output and in the subsequent differentiation of erythroid cells and megakaryocytes. An enhancer element upstream of the mouse Gata1 IE (1st exon erythroid) promoter, mHS−3.5, can direct both erythroid and megakaryocytic expression. However, loss of this element ablates only megakaryocytes, implying that an additional element has erythroid specificity. Here, we identify a double DNaseI hypersensitive site, mHS−25/6, as having erythroid but not megakaryocytic activity in primary cells. It binds an activating transcription factor complex in erythroid cells where it also makes physical contact with the Gata1 promoter. Deletion of mHS−25/6 or mHS−3.5 in embryonic stem cells has only a modest effect on in vitro erythroid differentiation, whereas loss of both elements ablates both primitive and definitive erythropoiesis with an almost complete loss of Gata1 expression. Surprisingly, Gata2 expression was also concomitantly low, suggesting a more complex interaction between these 2 factors than currently envisaged. Thus, whereas mHS−3.5 alone is sufficient for megakaryocytic development, mHS−3.5 and mHS−25/6 collectively regulate erythroid Gata1 expression, demonstrating lineage-specific differences in Gata1 cis-element use important for development of these 2 cell types.
Introduction
Vertebrate hematopoietic stem/progenitor cells generate 8 blood lineages and produce billions of new blood cells daily, most of which are erythrocytes. The complex cell fate choices required to generate blood cells are tightly regulated by extracellular signals and intracellular signaling cascades that modulate transcriptional activity of cell fate gene regulatory networks. Hematopoietic-specific regulators, such as the DNA-binding myeloid zinc finger protein GATA1, play a critical role in this process.1 The finding of mutations in GATA1 in patients with cytopenias and leukemia underlines its importance in human hematopoiesis (reviewed in Cantor,2 Vyas and Crispino,3 and Malinge et al4).
Gata1 expression is particularly important for proper differentiation and maturation of erythroid cells and megakaryocytes. GATA1-null mice die at approximately embryonic day 10.5 (E10.5) to E11.5 of anemia because of arrested primitive and definitive erythropoiesis.5 In vitro, GATA1-null murine embryonic stem (ES) cells fail to generate primitive erythroid cells; definitive erythroid progenitors are produced but undergo differentiation arrest and apoptosis at the proerythroblast stage.6 Megakaryocyte-specific loss of murine Gata1 expression blocks maturation,7 resulting in accumulation of abnormal megakaryocyte progenitors,8 hyperproliferation of immature megakaryocytes, and severe thrombocytopenia with platelet maturation defects.7,9 Moreover, ablation of Gata1 expression in adult mice also results in anemia and severe thrombocytopenia from a similar block in erythroid and megakaryocyte maturation as that seen in developmental hematopoiesis.10
Regulation of the level and spatiotemporal pattern of Gata1 transcription is critical during at least 2 key stages in hematopoiesis for orderly erythroid and megakaryocyte differentiation. In mouse, multipotential progenitor cells initiating Gata1 expression are committed to an erythroid/megakaryocyte (EM) fate.11 In contrast, those cells initiating Pu.1 expression are committed to a myeloid/lymphoid fate. Consistent with this, ectopic Gata1 or Pu.1 expression directs progenitors to EM or granulocyte-macrophage (GM) differentiation, respectively.12–15 Repression of Gata1 in zebrafish directs progenitors to differentiate to GM rather than EM cells, whereas repression of Pu.1 has the opposite effect.16,17 Furthermore, GATA1 and PU.1 mutually inhibit each other's transactivation function. Thus, initial expression of Gata1 and the relative level of GATA1 versus PU.1 critically determine initial commitment to the EM lineages.
Another key stage in which precise control of the pattern and levels of Gata1 expression is required is maturation of committed erythroid cells. Mouse mutants expressing Gata1 at 5%18 or 20%19 of normal levels, or conversely, increased levels of Gata120 die of anemia. Furthermore, the GATA1-null phenotype is rescued when an ectopic Gata1 gene is controlled by its own regulatory elements but not when it is controlled by the regulatory elements of another erythroid gene, β-globin, that has a different spatiotemporal expression pattern.21
To dissect the transcriptional control of mouse Gata1 expression we, and others, have identified Gata1 cis-regulatory elements. Initial studies showed that the gene is regulated by at least 3 cis-elements, each associated with a hematopoietic-specific DNaseI hypersensitive site (DHS): the hematopoietic promoter 1st exon erythroid (IE), an enhancer 3.5-kilobases (kb) upstream of the hematopoietic transcriptional start site (mHS−3.5, alternatively named HSI and G1HE), and an element 3.5 kb downstream from the IE promoter in the first Gata1 intron, mHS+3.5 (alternatively named HS4/5). In transgenic mice, mHS−3.5 is required for reporter gene expression in primitive red cells and megakaryocytes.22–24 mHS−3.5 and IE promoters also require mHS+3.5 for expression of reporter genes in definitive erythroid cells.23 Although these 3 elements drive gene expression in a pattern reminiscent of Gata1 expression, at least 3 lines of evidence suggest additional elements are involved in transcriptional regulation of Gata1. First, although mHS−3.5 is a major Gata1 enhancer, germline mHS−3.5 deletion (ΔmHS−3.5) ablates megakaryocyte but not erythroid Gata1 expression7; steady-state peripheral blood erythroid counts in ΔmHS−3.5 mice appear unaffected.19 Thus, mHS−3.5 has an essential nonredundant role for megakaryocyte Gata1 expression, whereas in erythropoiesis other elements are likely to compensate for its deletion. Second, reporter gene expression under the control of mHS−3.5, IE, and mHS+3.5 does not fully recapitulate erythroid Gata1 expression compared with gene expression from a bacterial artificial chromosome (BAC) that likely contains all Gata1 regulatory elements. In immature erythroid progenitors, only the BAC faithfully recapitulated Gata1 expression.25 Third, the 3 elements do not direct position-independent expression in transgenic mice.22–24
To identify other Gata1 regulatory elements, we systematically searched a 120-kb region of chromatin encompassing the human and mouse Gata1 loci.26 We identified a previously unrecognized mouse Gata1 cis-element 25 kb upstream of the IE promoter, associated with an erythroid-specific DHS with enhancer function in erythroid cell lines.26 This element is present in rodents and a potentially similar element is present 14 kb 3′ of the human GATA1 promoter.26 Here, we show that this mouse cis-element is present in primary erythroid cells and consists of 2 DHSs, collectively named mHS−25/6. Importantly, mHS−25/6 is an erythroid but not a megakaryocyte enhancer. Consistent with this, mHS−25/6 binds hematopoietic transcription factors in red cells but not megakaryocytes. Furthermore, the element is in physical proximity to the IE promoter in Gata1-expressing erythroid cells. In vitro differentiation of mutant ES cells shows that deletion of mHS−25/6 or mHS−3.5 has either a minimal or a modest impact on erythropoiesis, respectively, whereas compound deletion of the elements severely reduces Gata1 expression and ablates terminal erythroid differentiation. Our data suggest that the elements mHS−3.5 and mHS−25/6 collectively regulate erythroid Gata1 expression, whereas only mHS−3.5 is active in megakaryopoiesis.
Methods
DNaseI hypersensitive analysis
Nuclei preparation, DNaseI digestion, DNA extraction, and Southern blot analysis were performed as previously described.27
Transgenic procedures
To generate the reporter construct, an upstream primer 5′-GAGAGAGGTACCGCCATTGTTATTCAAACCACCG-3′ and a downstream primer 5′-GAGAGAGGTACCGCCTCTCAACTTGACCGTGG-3′ (Asp718 site underlined and the Gata1 locus–specific sequence in italics) were used to amplify genomic DNA. The amplified fragment extends from −26 050 to −24 700 relative to the IE promoter transcription start site. Polymerase chain reaction (PCR) products were then cloned into the Asp718 site of the 5′3′-LacZ vector.22 Standard techniques were used to isolate transgene sequences for DNA purification and for pronuclear injection of CD-1B6CBAF1/J (The Jackson Laboratory)–fertilized eggs. F0 transgenics were killed at E13.5 to E14.5, genotyped by PCR using LacZ and RapSyn primers as previously described,22 and analyzed for β-galactosidase expression as detailed in next paragraph.
β-Galactosidase assays
β-Galactosidase expression in fetal liver and bone marrow cells (E13.5-E14.5) was analyzed by flow cytometry using fluorescein di-(β-d-galactopyranoside) (FDG; Sigma-Aldrich).28 Lineage-specific staining was previously described.29 Biotin- or phycoerythrin (PE)–conjugated Ter119, Mac1, or CD61 or isotype controls were used in the stainings. Allophycocyanin-conjugated streptavidin was used as secondary antibody for biotin-conjugated primary antibodies. All antibodies were from BD Pharmingen. Fluorescence-activated cell sorting (FACS) analyses were performed using a CyAn machine and Summit software (Dako Cytomation). Erythroid cells were defined as Ter119+; megakaryocytes, as CD61+/Mac1−; and neutrophils/macrophages, as CD61−/Mac1+. Hoechst 33258 (Molecular Probes) was used to stain dead cells. A total of 500 000 to 800 000 events were counted.
ChIP analysis
Chromatin immunoprecipitation (ChIP) experiments on mouse primary megakaryocytes and erythroid cells from spleens of phenylhydrazine-injected mice were performed as previously described.26,30 Megakaryocytes (5 × 105) were used for each immunoprecipitation. All animal experiments were approved under a United Kingdom Home Office Project License.
Chromosome conformation capture
Chromosome conformation capture (3C) assays were performed using chromatin from fetal livers and brains of male C57BL6 embryos at E14.5, or mouse ES and induced MEL erythroid cells as described previously.31–33 Cells (1 × 107) were subjected to 2% formaldehyde and cross-linked nuclei were prepared. The cross-linked chromatin was digested overnight at 37°C with 400 U of EcoRI (606189; Roche). The following day, after inactivation of the restriction enzyme with 1.3% sodium dodecyl sulfate and incubation at 65°C for 25 minutes, the chromatin was ligated with 100 U of T4 DNA ligase (10799009001; Roche) for 4 hours at 16°C, followed by 30 minutes at room temperature. Samples were incubated overnight at 65°C to reverse the cross-links. The DNA was purified by phenol extraction and ethanol precipitation. DNA templates (200 ng) were used for Taqman/PCR reaction using normal PCR condition with ABI Prism 7000 (Applied Biosystems). PCR reactions, primers, and probes were optimized as described.33 Primers and probes were designed with a universal sequence-specific Taqman probe and reverse primer on a fixed restriction fragment in combination with different forward primers specific for other restriction fragments. All 3C results were normalized as previously described.33 Each PCR reaction was performed in duplicate and repeated 3 times.
Generation of the mHS−25/6 targeting construct
A targeting construct was made by recombineering. The construct contains a phosphoglycerate kinase–neomycin cassette flanked by LoxP sites and sequences homologous to 4.3 kb upstream and 5.9 kb downstream of mHS−25/6. The construct contains a negative selection cassette expressing the Thymidine kinase (TK) gene under control of the TK MC1 promoter.
Generation of recombinant ES clones
The targeting construct was linearized and electroporated into gelatin-adapted mouse AB2.2 (wild-type) ES cells34 or ΔmHS−3.5 J1 ES cells.7 Upon selection with G418 (0.3 mg/mL) and ganciclovir (0.2mM), resistant colonies were isolated and extensively characterized by Southern blotting, using various restriction enzymes/probes (not all shown). To excise the floxed Neomycin cassette, cells were transiently transfected with a vector expressing Cre-recombinase under control of the phosphoglycerate kinase promoter (kind gift from A. Green, University of Cambridge) by electroporation. G418-sensitive ES clones were checked for Neomycin excision by PCR and Southern blotting. ΔmHS−25/6 and mHS−25/6ΔmHS−3.5 cells with a normal karyotype were used for subsequent analysis.
In vitro differentiation
Previously described methods for in vitro hematopoietic differentiation of wild-type AB2.2 and J1 ES cells, ΔmHS−25/6, ΔmHS−3.5, and ΔmHS25/6ΔmHS−3.5 into embryoid bodies (EBs) and for secondary replating were used.35 Primary platings were repeated at least 6 times for each type of ES cells. To generate primitive and definitive erythroid colonies, EBs were disaggregated at days 6 and 8, respectively, and replated in methylcellulose in presence of erythropoietin (2 U/mL) and (only for definitive erythroid colonies) stem cell factor (100 ng/mL).
FACS for hematopoietic progenitors
EBs were disrupted at day 7 in 7.5mM ethylenediaminetetraacetic acid/phosphate-buffered saline for 1 minute and by passing through a 19-gauge needle. Samples were cleaned up by Ficoll treatment. Cells were labeled with rat anti–mouse antibodies directed against Gr-1 (553123), Mac-1 (553307), Ter119 (553671), B220 (553084), and interleukin-7 receptor (14-1271-85; eBioscience), then incubated with goat anti–rat immunoglobulin G Tricolour antibody (R40106; Caltag). Cells were blocked with 5% rat serum (Caltag) and stained with conjugated antibodies Sca-1–PE–cyanin 7 (558162), c-Kit–allophycocyanin (553356), CD34–fluorescein isothiocyanate (553733), and FcγIII/II–R-PE (553145). Dead cells were stained with 2 μg/mL propidium iodide. Cell populations were sorted with a MoFlow cell sorter (Dako Cytomation). Antibodies were from BD Pharmingen, unless stated differently.
Gene expression analysis
RNA from sorted cells was extracted with RNAeasy Micro RNA isolation kit (QIAGEN), and cDNA was synthesized using SuperScript III reverse transcriptase (Invitrogen). Ready-made primer and probe mixes were from Applied Biosystems. Gene expression was studied on an ABI Prism 7000 sequence detection system and normalized to Gapdh expression. Difference in gene expression between wild-type and ΔmHS−25/6ΔmHS−3.5 cell populations was analyzed by an unpaired parametric Student t test.
Results
DNaseI hypersensitive site analysis
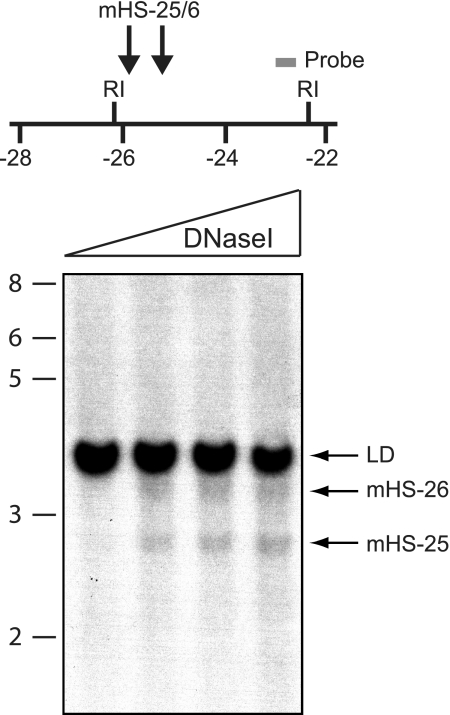
Our previous long-range DHS mapping of the Gata1 locus showed an element 25 kb upstream of the IE promoter in MEL cells.26 Here, we tested for the presence of this element in primary erythroid cells, using E14.5 fetal liver cells. DHS fine mapping shows that it is composed of 2 adjacent DHSs located approximately 25 and approximately 26 kb upstream of the IE promoter, hereafter collectively termed mHS−25/6 (Figure 1). Both sites were absent in Gata1-expressing primary eosinophils and in nonhematopoietic 3T3 fibroblast cell line.26 Furthermore, both sites were absent in the multipotential myeloid cell line FDCP-mix and in Gata1 nonexpressing primary neutrophils (data not shown). Collectively, these data suggest that this site may function specifically in erythroid cells.
Figure 1.
DNaseI hypersensitivity in primary erythroid cells. (Top) A portion of the Gata1 locus is shown. Coordinates (below the horizontal line) are in kilobases in respect to the Gata1 IE promoter. The black arrows show the position of mHS−25/6; the gray bar, the position of the probe used in the DNaseI hypersensitive mapping and R1, the position of EcoRI sites. (Below) Nuclei from E14.5 murine fetal liver cells were digested with increasing concentrations of DNaseI (triangle). No exogenous DNaseI was added to nuclei in the first lane. DNA was then extracted and digested with EcoRI and a Southern blot hybridized to the probe shown in the top panel. Size markers on the left are in kilobases. The blot shows 2 DHSs, mHS−25 and mHS−26, and the limited digest (LD).
mHS−25/6 is a functional erythroid enhancer
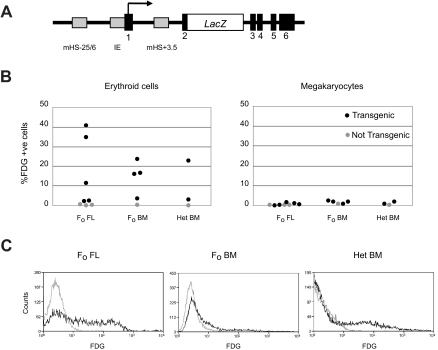
To perform enhancer activity studies in vivo, mHS−25/6 was attached to the LacZ reporter construct 5′3′LacZ.22 5′3′LacZ contains genomic DNA encompassing the IE promoter and the Gata1 gene including mHS+3.5 and has the β-galactosidase gene integrated in exon 2 at the first translational start site. We have previously shown that 5′3′LacZ is unable to express β-galactosidase in hematopoietic cells in transgenic mice.24 When a 1.6-kilobase (kb) fragment encompassing mHS−25/6 was attached to 5′3′LacZ (Figure 2A), β-galactosidase expression was detected in fetal liver erythroid cells of transgenic F0 embryos (range of expressing cells, 2.2%-40.7%; Figure 2B). In contrast, no significant expression was found in megakaryocytes of transgenic F0 embryos (Figure 2B). Similarly, in bone marrow of adult F0 and heterozygous transgenic mice, β-galactosidase expression was detected in erythroid cells (range of expressing cells, 2.9%-23.7%) but not in megakaryocytes (Figure 2B). Furthermore, no significant expression was found in granulocytes, B cells, and macrophages from bone marrow of F0 and heterozygous transgenic mice (data not shown). Figure 2C shows representative histograms of the distribution of FDG signal, which result from the expression of the β-galactosidase reporter gene, from erythroid cells of fetal liver and bone marrow of transgenic mice. Together, these data indicate that mHS−25/6 functions as an erythroid-specific enhancer in primary erythroid cells.
Figure 2.
Reporter gene expression driven by mHS−25/6 in transgenic mice. (A) Diagram of the reporter gene expression vector 5′3′LacZ to which a DNA fragment encompassing mHS−25/6 was attached. 5′3′LacZ consists of a genomic fragment that includes 2.3 kb of sequence upstream of the hematopoietic-specific IE promoter, IE (gray box), and 6 Gata1 exons (black boxes) and intervening introns. mHS+3.5 within the first intron is shown (red box). β-Galactosidase gene is fused in frame with the initiator ATG of the Gata1 gene. (B) Percentage of β-galactosidase expressing (%FDG+ve) TER-119+ erythroid cells (left) and CD61+Mac1− megakaryocytes (right) in F0 transgenic fetal livers (FL), F0 transgenic bone marrow (BM), and bone marrow for lines of mice heterozygous for the transgene (Het BM; black dots). Gray dots show percentage of FDG-positive cells in nontransgenic controls. (C) Representative FACS plots of FDG staining, demonstrating β-galactosidase expression, in erythroid populations from E14.5 transgenic F0 fetal liver (FL), transgenic F0 bone marrow (BM), and bone marrow for lines of mice heterozygous for the transgene (Het BM; black histogram). The gray histogram is from nontransgenic controls.
Hematopoietic transcription factor complex occupies mHS−25/6 in vivo
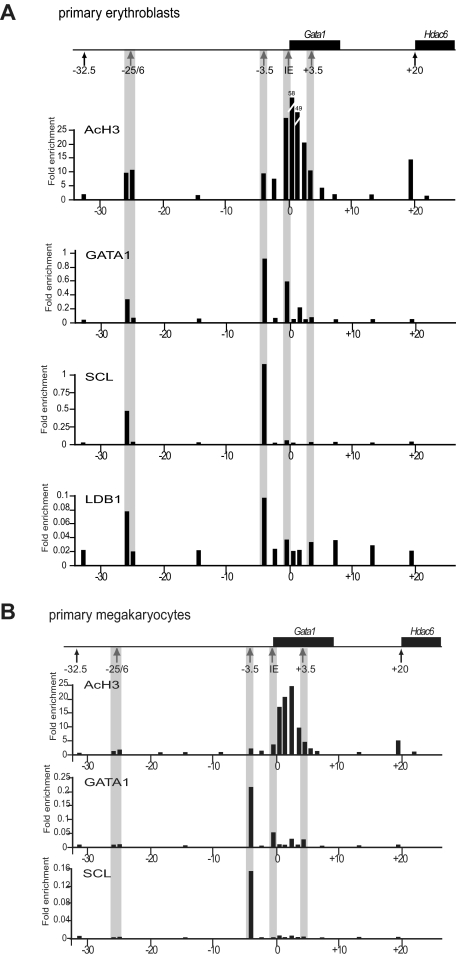
Consistent with erythroid enhancer activity of mHS−25/6, chromatin encompassing this element was enriched for acetylated histone H3 in primary erythroid cells and erythroid cell line MEL before and after induction of terminal erythroid differentiation (Figure 3A and supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Enrichment of acetylated H3 was also found with chromatin encompassing mHS−3.5, IE, the body of the Gata1 gene, and the promoter of the neighboring widely expressed gene Hdac6. Additional chromatin immunoprecipitation experiments showed that members of the hematopoietic pentameric transcription factor complex GATA1, SCL, and LDB1 bind to chromatin associated with mHS−25/6. Moreover, the binding appears to be specific for mHS−26. GATA1 binding was also detected at mHS−3.5 and at the IE promoter. Additional binding of SCL and LDB1 to the locus was restricted to mHS−3.5. Lack of SCL binding to the IE promoter is unexpected, because SCL has been reported to be a critical determinant of positive GATA1 activity.36
Figure 3.
Chromatin immunoprecipitation analysis in primary erythroblasts and megakaryoblasts. ChIP experiments were performed on primary erythroblasts (A) and megakaryocytes (B). The Gata1 locus is shown on top with the Gata1 and Hdac6 genes depicted as black boxes. Hematopoietic and general DNaseI hypersensitive sites are indicated with gray and black arrows, respectively. At various points along the Gata1 locus (x-axis, coordinates in kilobases with respect to the Gata1 transcriptional start site), the degree of enrichment of acetylated histone H3 (AcH3) GATA1, SCL, and LDB1 (in panel A only; black bars) is shown. Amplicons corresponding to mHS−25/6, mHS−3.5, IE promoter, and mHS+3.5 are highlighted by gray stripes. The 2 bars at position mHS−25/6 represent amplicons for mHS−25 and mHS−26 individually (right and left bars, respectively). Experiments were performed at least twice and data from representative experiments are shown.
In contrast, chromatin from primary megakaryocytes was not enriched, either for acetylated histone H3 or transcription factor binding, at mHS−25/6 (Figure 3B). Enrichment of acetylated H3 was found in the body of the Gata1 gene. GATA1 and SCL binding was detected only at mHS−3.5. Low level of GATA1 binding was detected at IE and mHS+3.5. Therefore, consistent with specific enhancer activity in erythroid cells, chromatin encompassing mHS−25/6 contains acetylated histones and is bound by GATA1, LDB1, and SCL in erythroid cells, but not in megakaryocytes.
Interaction between mHS−25/6 and the promoter in Gata1-expressing cells
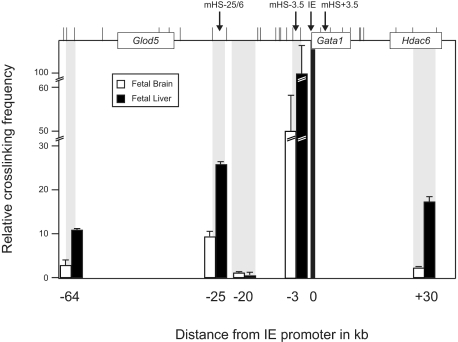
Physical interaction between enhancers and promoters has been shown to correlate with gene expression.31,37 We applied chromosome conformation capture (3C) to determine physical interactions between mHS−25/6 and the IE promoter. We compared interactions between these elements in E14.5 fetal liver cells and fetal brain cells (as Gata1 nonexpressing control; Figure 4). Because Gata1 is located on the X-chromosome, potential influence of X-chromosome silencing on cross-linking frequencies was avoided using only male cells. Cross-linking frequencies, an indication for proximity, between fragments containing the IE promoter and mHS−25/6 were markedly higher in fetal liver cells compared with fetal brain cells. Proximity of IE and a fragment between IE and mHS−25/6, namely −20, was significantly lower. The combination of these 2 observations suggests loop formation between mHS−25/6 and IE in erythroid cells. Cross-linking frequencies of the promoter and mHS−3.5 were high in both fetal liver and brain cells, explained by the linear proximity of the 2 elements.38 The proximity of IE to a fragment 64 kb upstream and 30 kb downstream were also higher in fetal liver cells than in fetal brain cells. These fragments encompass a ubiquitous DHS (64-kb upstream) and are part of the Hdac6 gene (30 kb), and these results would be consistent with the observation that linearly located active genes colocalize (F. Iborra, University of Oxford, oral communication). Similar results were found in cell lines, using MEL cells as Gata1-expressing erythroid cells and ES cells as a Gata1-nonexpressing control (supplemental Figure 2). Together, these data indicate that mHS−25/6 is in proximity of the IE promoter in Gata1-expressing erythroid cells.
Figure 4.
Intrachromosomal interactions between elements of the Gata1 locus. Cross-linking frequencies were determined using EcoRI-digested, fixed chromatin from E14.5 fetal livers (black bars) and fetal brains (white bars). Top of the figure is a schematic representation of the Gata1 locus with genes as boxes (Glod5, Gata1, and Hdac6). Cis-regulatory elements are indicated with arrows and vertical bars indicate EcoRI restriction sites. The figure shows relative cross-linking frequencies, as an indication of proximity, between various elements of the locus (gray bars) and the IE promoter (black bar). Each PCR was performed 3 times. Error bars denote SEM. Signals were normalized as previously described.33 Coordinates (in kilobases) of the DNA fragments analyzed are indicated on the x-axis.
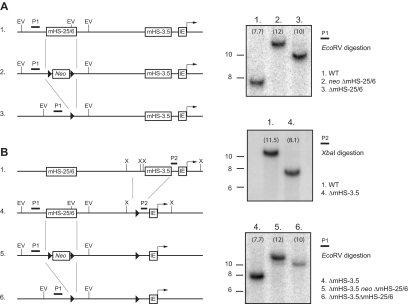
Establishment of ΔmHS−25/6 and ΔmHS−3.5ΔmHS−25/6 ES cells
To study the nonredundant role of mHS−25/6, a 2.2-kb fragment encompassing mHS−25/6 was replaced by a LoxP-flanked Neomycin gene in wild-type murine ES cells (Figure 5A). The targeting frequency was 4 of 248 clones. To test the hypothesis that mHS−3.5 and mHS−25/6 have overlapping functions in erythropoiesis, we also established ES cells with deletion of both mHS25/6 and mHS−3.5 by targeting ΔmHS−3.5 ES cells7 (Figure 5B). The targeting frequency was 1 of 166 clones. The Neomycin gene was excised in both single knockout (SKO) ΔmHS−25/6 and double knockout (DKO) ΔmHS−25/6ΔmHS−3.5 cells by transient Cre-recombinase expression. In the DKO ES cells, presence of the residual LoxP site at the -3.5 kb position sometimes resulted in excision of sequence between mHS−25/6 and mHS−3.5. However, we did not further analyze these mutant ES cell clones. All ES clones were checked for a normal karyotype.
Figure 5.
Establishment of ΔmHS−25/6 and ΔmHS−3.5ΔmHS−25/6 ES cells. The Gata1 locus is schematically depicted with mutations at various steps of the single and double knockout establishments. mHS−25/6 was replaced in wild-type (A) and in previously established ΔmHS−3.5 ES cells (B)19 by a LoxP (black triangles)–flanked neomycin gene. The neomycin gene was excised by transient Cre expression. Locations of restriction sites for EcoRV (EV) and XbaI (X) are shown. P1 and P2 show the location recognized by the probes used for Southern blots. On the right, examples of Southern blots confirming the various mutations. Numbers above the blots correspond with the loci on the left. Size markers on the left are in kilobases, and the numbers in the blots show the expected size of the detected DNA fragments.
Loss of cis-elements ablates primitive and definitive erythropoiesis
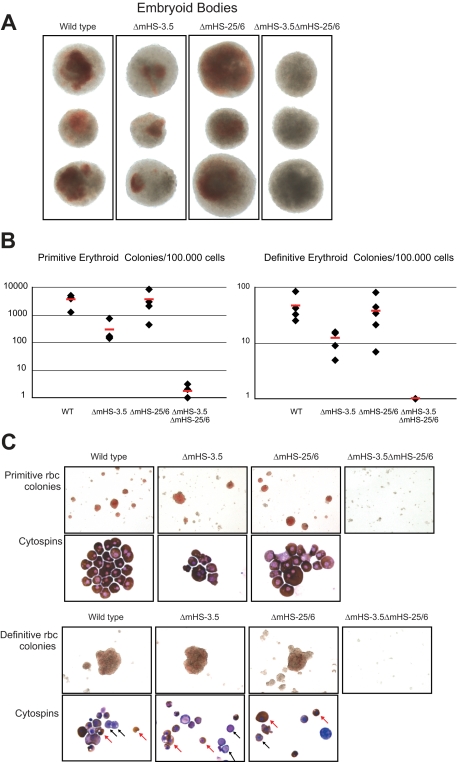
We studied the hematopoietic potential of ES cells with single and combined loss of mHS−3.5 and mHS−25/6 by 2-step in vitro differentiation assays.39 In the first step of the differentiation assay, embryoid bodies (EBs) with progenitors of hematopoietic lineages are formed. EBs from wild type and from both ΔmHS−3.5 and ΔmHS−25/6 SKO and DKO ES cells were assessed at day 8. Wild-type and SKO EBs had visible macroscopic hemoglobinization, judged by the appearance of red patches in the EBs (Figure 6A). In contrast, although DKO ES cells formed EBs of comparable size, they did not show visible hemoglobinization. To test the erythroid potential of mutant ES cells, EBs were dissociated at day 6 or 8 of differentiation and replated to enumerate primitive and definitive erythroid colonies, respectively (Figure 6B). Primitive colonies expressed predominantly the embryonic globin gene Hbb-y, whereas the definitive colonies expressed the definitive globin gene Hbb-b1 (data not shown). Replated cells from wild-type and ΔmHS−25/6 EBs produced a similar number of primitive and definitive erythroid colonies. Replated ΔmHS−3.5 EBs, on the other hand, produced 10-fold lower number of primitive colonies and 5-fold lower number of definitive colonies compared with wild-type controls. Colonies from ΔmHS−25/6 and ΔmHS−3.5 in vitro–differentiated ES cells contained primitive and definitive erythrocytes with normal morphology on May-Grünwald-Giemsa staining (Figure 6C).
Figure 6.
In vitro differentiation of ES cells. The phenotype of mutant ES cells was tested by 2-step in vitro differentiation. (A) Pictures of representative embryoid bodies at day 8 of differentiation. DKO ES cells form EBs, but lack macroscopic hemoglobinization as can be seen in wild-type and SKO EBs. (B) EBs were disrupted and replated to enumerate erythroid potential. Graphs show number of primitive (left) and definitive (right) erythroid colonies per 100 000 replated EB cells. Red bar indicates the average of colony numbers. (C) Pictures of representative primitive and definitive red blood cell (rbc) colonies of wild-type and mutant ES cells. ΔmHS−3.5ΔmHS−25/6 ES cells did not produce colonies. Below are pictures of cells from the erythroid colonies after cytospin and May-Grünwald-Giemsa/benzidine staining. Primitive erythroid cells of wild-type and SKO cells have a normal morphology. Cytospins from wild-type and SKO definitive erythroid colonies show cells at various stages of differentiation. Nonhemoglobinized progenitors are indicated with black arrows, and hemoglobinized (brown) erythroid cells are indicated by red arrows.
In marked contrast, ΔmHS−3.5ΔmHS−25/6 DKO ES cells did not generate primitive erythroid colonies. This is a phenocopy of GATA1-null ES cells, which also do not form primitive erythroid colonies in in vitro differentiation experiments.6 The DKO ES cells also did not generate definitive erythroid colonies. This phenotype surprisingly differs from GATA1-null ES cells, because they do form definitive erythroid colonies, albeit abnormal and lacking hemoglobin due to arrested differentiation6 (“Introduction” and “Discussion”). In summary, in vitro differentiation of ES cells demonstrates that loss of mHS−25/6 or mHS−3.5 has either a minimal or modest quantitative effect on erythroid progenitors and no obvious impact on terminal erythroid differentiation. However, loss of both elements ablates terminal primitive and definitive erythropoiesis.
Gene expression in hematopoietic progenitors
Next we wanted to test whether absence of erythroid colonies from differentiated ΔmHS−3.5ΔmHS−25/6 ES cells was consequent to aberrant Gata1 expression. As there are no erythroid colonies formed, to address this question we wished to study matched populations of myeloid progenitors from wild-type and DKO cells. However, to our knowledge, myeloid progenitors have not been prospectively isolated from EBs previously. Therefore, as a first step, we used published FACS protocols for isolating myeloid progenitors from fetal liver40 to obtain myeloid cell populations from dissociated wild-type day-7 EBs. A Lin−Sca1−c-Kit+ cell population was separated on CD34 and FcγR expression to obtain populations that immunophenotypically correspond to fetal liver common myeloid progenitors (CMPs), granulocyte-macrophage progenitors (GMPs), and megakaryocyte-erythroid progenitors (MEPs; supplemental Figure 3).
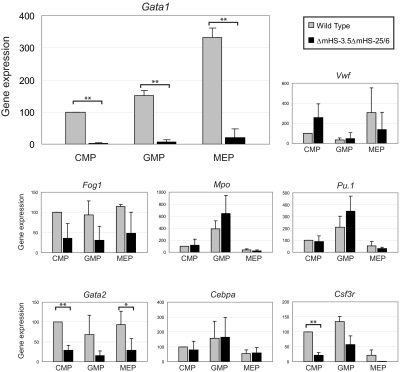
We next tested expression of lineage-specific genes in these sorted populations (Figure 7). Wild-type EB–derived populations with immunophenotypes corresponding to CMPs, GMPs, and MEPs showed patterns of gene expression commensurate with their expected lineage potentials. For example, the megakaryocyte/erythrocyte genes Gata1 and VWF showed higher expression in populations with an MEP compared with a GMP immunophenotype; VWF was expressed at a lower level in populations with a GMP compared with an MEP and a CMP immunophenotype (Figure 7). By contrast, the myeloid genes Pu.1, Mpo, Csf3r, and Cebpa were most highly expressed in populations with a GMP immunophenotype and expressed at a lower level in populations with an MEP compared with CMP immunophenotype. It is important to note that these wild-type populations did not give rise to colonies in methylcellulose assays (data not shown and “Discussion”). However, the immunophenotype and gene expression patterns of the sorted population are consistent with hematopoietic cells with the expected lineage potential and provide a tool to test Gata1 expression in DKO cell populations.
Figure 7.
Gene expression in hematopoietic progenitors. Gene expression in sorted hematopoietic populations from wild-type and DKO ES cells, as described in supplemental Figure 2, was measured by real-time PCR. Gene expression was normalized to Gapdh and levels in wild-type CMPs was set to 100%. Experiments were performed 3-5 times and error bars indicate the standard deviation. We tested expression of genes coding for GATA1, VWF, FOG1, GATA2, MPO, C/EBPα, PU.1, and G-CSF-R. Difference in expression between paired hematopoietic populations from wild-type and DKO ES cells was significant at **P < .005 or *P < .05.
Immunophenotypic staining of cells from DKO EBs gave similar population as from WT (supplemental Figure 3), suggesting that deletion of mHS−3.5mHS−25/6 does not significantly alter either formation of these populations or antigen expression. Importantly, gene expression analysis showed that in all 3 DKO populations, Gata1 expression was drastically reduced to approximately 5% of wild-type levels (Figure 7). In concordance with this, expression of Fog1, a direct GATA1 target gene,41 was lower in the DKO compared with wild-type populations. Interestingly, expression of Gata2 in the DKO was also reduced (“Discussion”). The pattern of VWF expression in the DKO populations was also deregulated; unlike expression in wild-type populations, the immunophenotypic MEPs did not show higher VWF expression than the immunophenotypic CMPs. However, the expression pattern and level of the control myeloid genes Mpo, Cebpa, and Pu.1, were comparable in populations from DKO and wild-type EBs. The pattern of Csf3r (G-CSF receptor) expression was also similar between DKO and wild-type EBs, although the level was lower in DKO populations. This suggests that granulocyte-macrophage priming may not have been altered in DKO EB–derived populations. Taken together, these data show that in absence of both mHS−3.5 and mHS−25/6, Gata1 expression is markedly reduced in EB-derived immunophenotypic hematopoietic progenitor populations.
Discussion
Tight spatiotemporal regulation of Gata1 expression is required for proper myeloid lineage specification and terminal differentiation of erythroid cells and megakaryocytes. Important Gata1 cis-regulatory elements include mHS−3.5, mHS+3.5, and the IE promoter. Previously, we have searched for other potential cis-regulatory elements26 and here we present data that mHS−25/6 is also a bona fide Gata1 in vivo erythroid enhancer. It drives reporter gene expression specifically in erythroid cells in transgenic mice. It makes physical contact with the IE promoter in primary erythroid cells. Chromatin associated with mHS−25/6 forms erythroid-specific DHS, is enriched for acetylated histone H3, and binds a hematopoietic transcription factor complex that includes GATA1, SCL, and LDB1. Such a complex has been shown to activate gene expression in erythroid cells.36,42 During MEL cell differentiation, Gata1 expression, acetylation, and GATA1 binding at mHS−25/6 do not change (supplemental Figure 1). In contrast, SCL and LDB1 binding did fall at HS−25/26. Although preliminary data suggest that both mHS−25 and mHS−26 have enhancer activity in transgenic reporter assays, all 3 transcription factors specifically bind mHS−26, possibly indicating different functions for the 2 elements. Further detailed functional analysis of mHS−25 and mHS−26 in transgenic assays where the elements are integrated into the same genomic location (eg, the Hprt locus) may reveal specific functions of mHS−25 and mHS−26. In this paper, we have focused on their combined function.
We then addressed a key question: what are the nonredundant and overlapping in vivo roles of mHS−25/6 and mHS−3.5? Despite clear evidence that mHS−25/6 has erythroid enhancer activity, erythroid differentiation of ΔmHS−25/6 ES cells is well preserved. It is likely that mHS−3.5 compensates for loss of mHS−25/6. However, given that mHS−3.5 (together with IE promoter and mHS+3.5) does not fully recapitulate the levels or pattern of reporter gene expression during erythropoiesis compared with BAC reporter construct containing 196 kb of the Gata1 locus (containing mHS−25/6),25 it is unclear whether mHS−3.5 will fully compensate for loss of mHS−25/6 in vivo. To address this, analysis of steady state and stressed erythropoiesis and Gata1 expression in ΔmHS−25/6 mutant animals is required.
In contrast to ΔmHS−25/6 ES cells, ΔmHS−3.5 ES cells have a significantly reduced primitive and definitive erythroid colony formation. Although initial studies of ΔmHS−3.5 mice suggested an absence of an erythroid defect (these mice have a normal steady-state hemoglobin and fetal liver erythroid colony output19), recent more detailed analysis of the same mutant strain shows a 75% reduction of Gata1 mRNA levels in early erythroid progenitors (erythroid burst-forming unit stage), resulting in an accumulation of early erythroid progenitors. There was also 50% reduction of Gata1 mRNA levels in later erythroid progenitors (erythroid colony-forming unit stage), but this level of Gata1 expression appears to be sufficient for terminal erythroid maturation.25
Our observation here that compound deletion of mHS−25/6 and mHS−3.5 ablates terminal erythropoiesis is in contrast to the milder erythroid phenotypes of SKO ES cells and supports the notion that mHS−25/6 and mHS−3.5 have overlapping roles in directing Gata1 expression. This raises 2 questions. First, what effect does the combined deletion have on Gata1 expression? Second, why does the erythroid phenotype of the DKO ES cells differ from that of GATA1-null ES cells?
Given the absence of erythroid cells from DKO ES cells, we isolated populations of FACS-sorted matched myeloid populations with the same immunophenotype as previously described for fetal liver CMPs, GMPs, and MEPs40 from dissociated wild-type and DKO day-7 EBs to test whether absence of terminal erythropoiesis is preceded by aberrant Gata1 expression. The antigen staining patterns of c-kit, Sca1, CD34, and FcγR from wild-type and DKO EBs were comparable with each other and with those in fetal liver (data not shown and Traver et al40). Moreover, these EB-derived populations expressed several hematopoietic genes in a pattern consistent with CMPs, GMPs, and MEPs.11 Importantly, Gata1 expression was severely reduced in all 3 DKO populations compared with wild type. As we tested gene expression in immunophenotypically similar wild-type and DKO populations, the difference in Gata1 expression does not reflect absence of mature erythroid cells in DKO EBs. An important caveat to these results is that even wild-type EB-derived populations did not form colonies in methylcellulose progenitor assays. Possible reasons for this include either inefficiency of methylcellulose colony assays to read out EB progenitors or sensitivity of EB-derived sorted populations to withstand dissociation, immunostaining, and FACS.
The strong reduction of Gata1 expression in DKO cells can explain the absence of primitive colony formation because it is similar to GATA1-null ES cells.6 However, the absence of definitive erythroid colonies from DKO ES cells differs from the poorly hemoglobinized colony growth from in vitro–differentiated GATA1-null ES cells.6 There are at least 2 possible explanations for the different phenotypes. First, DKO ES cells may have acquired additional genetic or epigenetic changes that compromise their ability to form colonies in vitro. Arguments against this are that the DKO ES cells have a normal karyotype, form EBs and cell populations with the immunophenotype of hematopoietic progenitors, and express hematopoietic genes. An alternative possibility is, because the differentiating ΔmHS−25/6ΔmHS−3.5 cells have reduced expression of both Gata1 and Gata2, the total GATA factor concentration may be too low to support erythroid cell proliferation and colony formation. In support of this hypothesis (a) reduced Gata2 expression severely reduces proliferation of definitive hematopoietic cells and erythroid colony formation by 95%,43 (b) GATA1-null arrested proerythroblast cells have a high level of GATA2,6 and (c) mice deleted for both Gata1 and Gata244 have a more severe phenotype than either GATA1-null or GATA2-null mice, with complete absence of primitive and definitive erythropoiesis.5,43,44 To explore this hypothesis, we tested the erythroid potential of GATA1/GATA2-null ES cells (kind gift from Prof S. H. Orkin, Dana-Farber Cancer Institute) by in vitro differentiation. Similar to ΔmHS−25/6ΔmHS−3/5 ES cells, these ES cells did not form primitive or definitive erythroid colonies (data not shown).
Finally, the observation of reduced Gata2 expression in ΔmHS−25/6ΔmHS−3/5 cells, where Gata1 expression is low, is contrary to the high level of Gata2 expression seen in GATA1-null terminally maturing erythroid cells.6 One possible explanation for this difference is that we have measured Gata1 and Gata2 expression in cell populations that are much earlier in hematopoiesis than proerythroblasts. In these early populations, we suggest that low Gata1 expression (as a primary event from targeting the Gata1 locus) could lead to low Gata2 expression. Thus, we would speculate that GATA1 positively regulates/maintains Gata2 expression in early myeloid progenitor cells before the proerythroblast stage. This refines the current model of GATA factor cross regulation in early cells, where only GATA2 promotes Gata1 expression and suggests GATA1 may not just lie downstream of GATA2. The more general point of this work is that it demonstrates that one way to dissect complex transcriptional regulation between key regulators to generate accurate network models of cell fate control is by studying allelic series of mutants lacking cis-elements of key transcriptional regulators.
Acknowledgments
We thank Alan Bradley and Pentao Lui (Sanger Institute) for AB2.2 ES cells and recombineering vectors, and Stuart Orkin (Dana-Farber Cancer Institute) for ΔmHS−3.5 ES cells. We thank Hedia Chagraoui, Douglas Vernimmen, Juan Li, and Rita Ferreira for help with design and execution of experiments. We thank Douglas Higgs and Sjaak Philipsen for intellectual input.
This work was funded by the Oxford Partnership Comprehensive Biomedical Research Centre with funding from the Department of Health's National Institutes for Health Research Biomedical Research Centers funding scheme. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the paper.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: R.D., B.G., L.Z., and A.A. designed and performed research and analyzed data; J.S.-S. and B.W. performed research and analyzed data; C.P. and P.V. designed research and analyzed data; and all contributors helped to write the paper.
Conflict-of-interest disclosure: R.D., B.G., and P.V. were funded by Wellcome Trust and the Medical Research Council; L.Z., J.S.-S., B.W., and C.P. were supported by Medical Research Council. The remaining author declares no competing financial interests.
Correspondence: Paresh Vyas, MRC Molecular Haematology Unit and Department of Haematology, Weatherall Institute of Molecular Medicine, John Radcliffe Hospital, Oxford OX3 9DU, United Kingdom; e-mail: paresh.vyas@imm.ox.ac.uk.
References
- 1.Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132(4):631–644. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cantor AB. GATA transcription factors in hematologic disease. Int J Hematol. 2005;81(5):378–384. doi: 10.1532/ijh97.04180. [DOI] [PubMed] [Google Scholar]
- 3.Vyas P, Crispino JD. Molecular insights into Down syndrome-associated leukemia. Curr Opin Pediatr. 2007;19(1):9–14. doi: 10.1097/MOP.0b013e328013e7b2. [DOI] [PubMed] [Google Scholar]
- 4.Malinge S, Izraeli S, Crispino JD. Insights into the manifestations, outcomes, and mechanisms of leukemogenesis in Down syndrome. Blood. 2009;113(12):2619–2628. doi: 10.1182/blood-2008-11-163501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fujiwara Y, Browne CP, Cunniff K, Goff SC, Orkin SH. Arrested development of embryonic red cell precursors in mouse embryos lacking transcription factor GATA-1. Proc Natl Acad Sci U S A. 1996;93(22):12355–12358. doi: 10.1073/pnas.93.22.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weiss MJ, Keller G, Orkin SH. Novel insights into erythroid development revealed through in vitro differentiation of GATA-1 embryonic stem cells. Genes Dev. 1994;8(10):1184–1197. doi: 10.1101/gad.8.10.1184. [DOI] [PubMed] [Google Scholar]
- 7.Shivdasani RA, Fujiwara Y, McDevitt MA, Orkin SH. A lineage-selective knockout establishes the critical role of transcription factor GATA-1 in megakaryocyte growth and platelet development. EMBO J. 1997;16(13):3965–3973. doi: 10.1093/emboj/16.13.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuhl C, Atzberger A, Iborra F, Nieswandt B, Porcher C, Vyas P. GATA1-mediated megakaryocyte differentiation and growth control can be uncoupled and mapped to different domains in GATA1. Mol Cell Biol. 2005;25(19):8592–8606. doi: 10.1128/MCB.25.19.8592-8606.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vyas P, Ault K, Jackson CW, Orkin SH, Shivdasani RA. Consequences of GATA-1 deficiency in megakaryocytes and platelets. Blood. 1999;93(9):2867–2875. [PubMed] [Google Scholar]
- 10.Gutiérrez L, Tsukamoto S, Suzuki M, et al. Ablation of Gata1 in adult mice results in aplastic crisis, revealing its essential role in steady-state and stress erythropoiesis. Blood. 2008;111(8):4375–4385. doi: 10.1182/blood-2007-09-115121. [DOI] [PubMed] [Google Scholar]
- 11.Arinobu Y, Mizuno S, Chong Y, et al. Reciprocal activation of GATA-1 and PU. 1 marks initial specification of hematopoietic stem cells into myeloerythroid and myelolymphoid lineages. Cell Stem Cell. 2007;1(4):416–427. doi: 10.1016/j.stem.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Kulessa H, Frampton J, Graf T. GATA-1 reprograms avian myelomonocytic cell lines into eosinophils, thromboblasts, and erythroblasts. Genes Dev. 1995;9(10):1250–1262. doi: 10.1101/gad.9.10.1250. [DOI] [PubMed] [Google Scholar]
- 13.Nerlov C, Graf T. PU. 1 induces myeloid lineage commitment in multipotent hematopoietic progenitors. Genes Dev. 1998;12(15):2403–2412. doi: 10.1101/gad.12.15.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heyworth C, Pearson S, May G, Enver T. Transcription factor-mediated lineage switching reveals plasticity in primary committed progenitor cells. EMBO J. 2002;21(14):3770–3781. doi: 10.1093/emboj/cdf368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwasaki H, Mizuno S, Wells RA, Cantor AB, Watanabe S, Akashi K. GATA-1 converts lymphoid and myelomonocytic progenitors into the megakaryocyte/erythrocyte lineages. Immunity. 2003;19(3):451–462. doi: 10.1016/s1074-7613(03)00242-5. [DOI] [PubMed] [Google Scholar]
- 16.Galloway JL, Wingert RA, Thisse C, Thisse B, Zon LI. Loss of gata1 but not gata2 converts erythropoiesis to myelopoiesis in zebrafish embryos. Dev Cell. 2005;8(1):109–116. doi: 10.1016/j.devcel.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Rhodes J, Hagen A, Hsu K, et al. Interplay of pu.1 and gata1 determines myelo-erythroid progenitor cell fate in zebrafish. Dev Cell. 2005;8(1):97–108. doi: 10.1016/j.devcel.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi S, Onodera K, Motohashi H, et al. Arrest in primitive erythroid cell development caused by promoter-specific disruption of the GATA-1 gene. J Biol Chem. 1997;272(19):12611–12615. doi: 10.1074/jbc.272.19.12611. [DOI] [PubMed] [Google Scholar]
- 19.McDevitt MA, Shivdasani RA, Fujiwara Y, Yang H, Orkin SH. A “knockdown” mutation created by cis-element gene targeting reveals the dependence of erythroid cell maturation on the level of transcription factor GATA-1. Proc Natl Acad Sci U S A. 1997;94(13):6781–6785. doi: 10.1073/pnas.94.13.6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whyatt D, Lindeboom F, Karis A, et al. An intrinsic but cell-nonautonomous defect in GATA-1-overexpressing mouse erythroid cells. Nature. 2000;406(6795):519–524. doi: 10.1038/35020086. [DOI] [PubMed] [Google Scholar]
- 21.Ferreira R, Wai A, Shimizu R, et al. Dynamic regulation of Gata factor levels is more important than their identity. Blood. 2007;109(12):5481–5490. doi: 10.1182/blood-2006-11-060491. [DOI] [PubMed] [Google Scholar]
- 22.McDevitt MA, Fujiwara Y, Shivdasani RA, Orkin SH. An upstream, DNase I hypersensitive region of the hematopoietic-expressed transcription factor GATA-1 gene confers developmental specificity in transgenic mice. Proc Natl Acad Sci U S A. 1997;94(15):7976–7981. doi: 10.1073/pnas.94.15.7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Onodera K, Takahashi S, Nishimura S, et al. GATA-1 transcription is controlled by distinct regulatory mechanisms during primitive and definitive erythropoiesis. Proc Natl Acad Sci U S A. 1997;94(9):4487–4492. doi: 10.1073/pnas.94.9.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vyas P, McDevitt MA, Cantor AB, Katz SG, Fujiwara Y, Orkin SH. Different sequence requirements for expression in erythroid and megakaryocytic cells within a regulatory element upstream of the GATA-1 gene. Development. 1999;126(12):2799–2811. doi: 10.1242/dev.126.12.2799. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki M, Moriguchi T, Ohneda K, Yamamoto M. Differential contribution of the Gata1 gene hematopoietic enhancer to erythroid differentiation. Mol Cell Biol. 2009;29(5):1163–1175. doi: 10.1128/MCB.01572-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valverde-Garduno V, Guyot B, Anguita E, Hamlett I, Porcher C, Vyas P. Differences in the chromatin structure and cis-element organization of the human and mouse GATA1 loci: implications for cis-element identification. Blood. 2004;104(10):3106–3116. doi: 10.1182/blood-2004-04-1333. [DOI] [PubMed] [Google Scholar]
- 27.Vyas P, Vickers MA, Simmons DL, Ayyub H, Craddock CF, Higgs DR. Cis-acting sequences regulating expression of the human alpha-globin cluster lie within constitutively open chromatin. Cell. 1992;69(5):781–793. doi: 10.1016/0092-8674(92)90290-s. [DOI] [PubMed] [Google Scholar]
- 28.Fiering SN, Roederer M, Nolan GP, Micklem DR, Parks DR, Herzenberg LA. Improved FACS-Gal: flow cytometric analysis and sorting of viable eukaryotic cells expressing reporter gene constructs. Cytometry. 1991;12(4):291–301. doi: 10.1002/cyto.990120402. [DOI] [PubMed] [Google Scholar]
- 29.Guyot B, Murai K, Fujiwara Y, et al. Characterization of a megakaryocyte-specific enhancer of the key hemopoietic transcription factor GATA1. J Biol Chem. 2006;281(19):13733–13742. doi: 10.1074/jbc.M602052200. [DOI] [PubMed] [Google Scholar]
- 30.Guyot B, Valverde-Garduno V, Porcher C, Vyas P. Deletion of the major GATA1 enhancer HS 1 does not affect eosinophil GATA1 expression and eosinophil differentiation. Blood. 2004;104(1):89–91. doi: 10.1182/blood-2004-01-0108. [DOI] [PubMed] [Google Scholar]
- 31.Drissen R, Palstra RJ, Gillemans N, et al. The active spatial organization of the beta-globin locus requires the transcription factor EKLF. Genes Dev. 2004;18(20):2485–2490. doi: 10.1101/gad.317004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Splinter E, Grosveld F, de Laat W. 3C technology: analyzing the spatial organization of genomic loci in vivo. Methods Enzymol. 2004;375:493–507. doi: 10.1016/s0076-6879(03)75030-7. [DOI] [PubMed] [Google Scholar]
- 33.Vernimmen D, De Gobbi M, Sloane-Stanley JA, Wood WG, Higgs DR. Long-range chromosomal interactions regulate the timing of the transition between poised and active gene expression. EMBO J. 2007;26(8):2041–2051. doi: 10.1038/sj.emboj.7601654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramírez-Solis R, Liu P, Bradley A. Chromosome engineering in mice. Nature. 1995;378(6558):720–724. doi: 10.1038/378720a0. [DOI] [PubMed] [Google Scholar]
- 35.Porcher C, Swat W, Rockwell K, Fujiwara Y, Alt FW, Orkin SH. The T cell leukemia oncoprotein SCL/tal-1 is essential for development of all hematopoietic lineages. Cell. 1996;86(1):47–57. doi: 10.1016/s0092-8674(00)80076-8. [DOI] [PubMed] [Google Scholar]
- 36.Tripic T, Deng W, Cheng Y, et al. SCL and associated proteins distinguish active from repressive GATA transcription factor complexes. Blood. 2009;113(10):2191–2201. doi: 10.1182/blood-2008-07-169417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palstra RJ, Tolhuis B, Splinter E, Nijmeijer R, Grosveld F, de Laat W. The beta-globin nuclear compartment in development and erythroid differentiation. Nat Genet. 2003;35(2):190–194. doi: 10.1038/ng1244. [DOI] [PubMed] [Google Scholar]
- 38.Tolhuis B, Palstra RJ, Splinter E, Grosveld F, de Laat W. Looping and interaction between hypersensitive sites in the active beta-globin locus. Mol Cell. 2002;10(6):1453–1465. doi: 10.1016/s1097-2765(02)00781-5. [DOI] [PubMed] [Google Scholar]
- 39.Keller G, Kennedy M, Papayannopoulou T, Wiles MV. Hematopoietic commitment during embryonic stem cell differentiation in culture. Mol Cell Biol. 1993;13(1):473–486. doi: 10.1128/mcb.13.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Traver D, Miyamoto T, Christensen J, Iwasaki-Arai J, Akashi K, Weissman IL. Fetal liver myelopoiesis occurs through distinct, prospectively isolatable progenitor subsets. Blood. 2001;98(3):627–635. doi: 10.1182/blood.v98.3.627. [DOI] [PubMed] [Google Scholar]
- 41.Welch JJ, Watts JA, Vakoc CR, et al. Global regulation of erythroid gene expression by transcription factor GATA-1. Blood. 2004;104(10):3136–3147. doi: 10.1182/blood-2004-04-1603. [DOI] [PubMed] [Google Scholar]
- 42.Schuh AH, Tipping AJ, Clark AJ, et al. ETO-2 associates with SCL in erythroid cells and megakaryocytes and provides repressor functions in erythropoiesis. Mol Cell Biol. 2005;25(23):10235–10250. doi: 10.1128/MCB.25.23.10235-10250.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsai FY, Keller G, Kuo FC, et al. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature. 1994;371(6494):221–226. doi: 10.1038/371221a0. [DOI] [PubMed] [Google Scholar]
- 44.Fujiwara Y, Chang AN, Williams AM, Orkin SH. Functional overlap of GATA-1 and GATA-2 in primitive hematopoietic development. Blood. 2004;103(2):583–585. doi: 10.1182/blood-2003-08-2870. [DOI] [PubMed] [Google Scholar]