Left ventricular (LV) remodeling, defined as changes in myocardial structure and geometry, is considered to be a fundamental milestone in the progression to heart failure. This is particularly true in the clinical context of a myocardial infarction (MI) whereby adverse LV remodeling is directly associated with the progression to heart failure and increased morbidity and mortality.1 LV remodeling is a multifactorial process which includes changes within the myocardial, vascular and extracellular matrix (ECM). However, in terms of post-MI remodeling, changes in myocardial growth/viability and ECM structure/composition are ubiquitous events, and occur in a heterogeneous fashion within the remote viable myocardium, the myocardial region surrounding the MI (borderzone), and the MI scar itself. It is the summation of the alterations within both the cellular and extracellular compartments, which occur in all these regions post-MI, which promulgate adverse LV remodeling and ultimately the progression to heart failure. Through the use of animal models and clinical translational studies, certain signaling and proteolytic events have been identified to uniformly occur following an MI, and likely induce the cascade of events which yield changes in myocardial structure and function.2–8 In this issue of Circulation, Jugdutt and colleagues report how activation of a specific signaling pathway, the angiotensin-II receptor, influences a number of critical cellular signaling and ECM proteolytic events which can contribute to adverse LV remodeling.2 These investigators examined a number of critical pathways following a period of ischemia and reperfusion, which resulted in a significant and relevant MI. These investigators not only examined these signaling/proteolytic events in a clinically relevant model of MI, but more importantly examined the post-MI remodeling process within the aging myocardium. The findings from this study are important for 2 reasons. First, this study clearly demonstrated that important interactions occurred between the angiotensin-II receptor and the induction of bioactive molecules and proteases following an acute MI. Second, this study demonstrated that an amplified response occurs between these intersecting pathways within the aging myocardium following an acute MI. Taken together, the findings by Jugdutt and colleagues provide mechanistic evidence that the elderly myocardium is a more vulnerable substrate to an ischemic insult, and that this is likely due to enhanced/amplified induction of signaling cascades and proteolytic events that would directly contribute to a more advanced and accelerated LV remodeling process.
Myocardial Cell Death Following MI and with Aging
Myocyte loss following MI can occur through 3 different mechanisms: necrosis, apoptosis and defects in autophagy.6,9 While the first mechanism contributes to the acute and significant loss of viable myocytes, it is likely that these other pathways contribute to continued myocyte cell death long after the acute event and can contribute to progressive LV remodeling. Alterations in apoptosis and autophagy have been identified with aging,6,9 which would lead to the hypothesis that the aging myocardium when subjected to a similar stress/insult is more susceptible to adverse LV remodeling than that of young myocardium. Indeed, an initial proteomic approach in aging mice identified alterations in levels of critical proteins involved in modulating oxidative stress.10 Jugdutt and colleagues identified that inducible nitric oxide synthase (iNOS) was robustly increased within the aging myocardium following an acute MI, which was paralleled by an induction of inflammatory mediators such as interleukin-6 (IL-6) and tumor necrosis factor (TNF).2 In these studies, infusion of an angiotensin-II receptor antagonist at the time of reperfusion significant reduced these markers of oxidative stress and cytokine activation. Whether and to what degree infusion of an angiotensin-II receptor antagonist at the time of the initial MI would prevent myocyte loss/remodeling through mediating apoptotic/autophagic pathways, and in turn cause a long term favorable effect on LV remodeling remains to be established. Nevertheless, this large animal model provides additional evidence that a “priming effect” exists within the senescent myocardium that would cause a robust increase in signaling pathways which contribute to adverse LV remodeling.
Myocardial ECM Following MI and with Aging
Degradation of the ECM following the acute phase of an MI is considered to be an essential event which allows for the ingress of inflammatory cells, proliferation and maturation of macrophages and fibroblasts, and provides the necessary substructure for scar formation. In the early part of the 20th century, it was identified that early (within 24 hours) following an MI, degradation of the normal collagen matrix occurred, which was then followed by significant matrix deposition.11 It is now recognized that the myocardial ECM is a complex microenvironment containing a large portfolio of matrix proteins, signaling molecules, proteases, and cell types that play a fundamental role in the post-MI remodeling process. In terms of the structure and composition of the ECM, a hallmark feature of the aging myocardium is increased collagen accumulation, which in turn impairs myocardial compliance and diastolic function. With respect to ECM signaling molecules, a pleotropic bioactive molecule which plays a predominant role in regulation ECM synthesis, is transforming growth factor (TGF). Alterations in the response of the aging myocardium to TGF, particularly following an MI have been reported.4,8 The matricellular protein, osteopontin is another bioactive molecule which can induce a potent pro-fibrotic response both in-vitro and in-vivo.5 Both the TGF and opsteopontin signaling pathways can be significantly influenced by angiotensin-II.4,5 The biosynthesis of ECM proteins, such as collagens require a series of post-translational steps which include appropriate localization, proteolytic processing, and ultimately enzyme mediated maturation and cross-linking. One of the matricellular proteins which has been recognized to play a role in post-translational processing of the ECM, and can contribute to adverse matrix remodeling with aging, is the secreted protein acidic and rich in cysteine (SPARC).12 SPARC facilitates the formation of mature collagen fibrils and deletion of this matricellular protein can reduce collagen cross-linking and myocardial stiffness, particularly in aged mice.12 The study by Jugdutt provides further demonstration of the interaction between angiotensin-II receptor and the induction of profibrotic signaling molecules and matricellular proteins during the acute phase of MI.
In addition to the pro-synthetic ECM pathways which are stimulated following an MI, induction, activation and release of ECM proteolytic enzymes occur which include serine proteases, matrix metalloproteinases (MMPs) and a-disintegrin- and-metalloproteinases (ADAMs). With respect to the MMPs, this constitutes a large family of proteolytic enzymes, and while the role of each of these MMPs is just beginning to be elucidated, a signature of certain MMP types appear to be released following an acute MI in patients and include the soluble MMP types MMP-2 and MMP-9.3,7 While the predominant pathway for MMP-2 activation is likely through another MMP type, the membrane type-1 MMP,3 an alternative activation mechanism which is likely to be operative in the context of acute tissue injury and inflammation, is through proteolytic processing by serine proteases. Inflammatory cells such as macrophages and neutrophils release certain MMP types such as MMP-9, serine proteases and inhibitors of serine proteases such as the secretory-leukocyte-protease-inhibitor (SLPI).13 In past animal studies, it has been shown that SLPI can terminate the acute inflammatory mediated proteolytic response and facilitate wound healing.13 In the study by Jugdutt and colleagues, increased levels of proteolytic enzymes such as ADAM-10, -17, and MMP-2 as well as SLPI were reported in the aging myocardium, and in turn would likely yield alterations in ECM homeostasis under steady state conditions as well as following acute MI.
Aging, Myocardial Remodeling and Basic Research; A Paradox
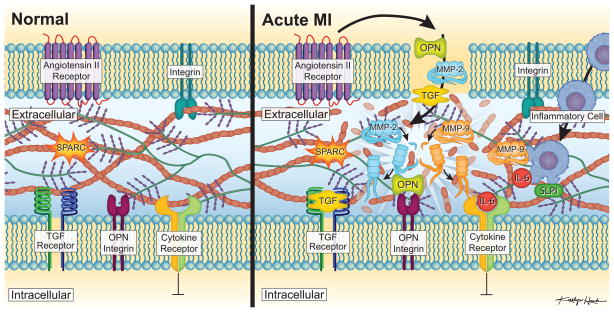
Cardiovascular disease, in particular ischemic heart disease is a function of age, where the incidence increases in almost an exponential fashion following the 5th decade of life (Heart disease and stroke statistics – 2010 update. American Heart Association, 2010. http://www.americanheart.org/downloadable/heart/12626426574432010%20Stat%20charts%20FINAL.ppt). Clinically, it is well recognized that elderly patients can be at much higher risk for LV remodeling and the progression to heart failure, despite an equivalent initial MI size when compared to younger cohorts of patients. The preponderance of basic research over the past decade in terms of mechanisms and pathways which promulgate LV remodeling post-MI have been performed in young mice using transgenic approaches. However, biological signaling pathways, proteolytic portfolios and the overall response to myocardial injury can be quite different in these small, young rodents when compared to larger mammals.4,5,14 While these murine studies have provided invaluable insight and provoke new hypotheses, these must be carried forward through the use of large animals which more closely recapitulate the clinically relevant context. Jugdutt and colleagues used a large animal model of aging and MI which demonstrated the complexity of the ECM with respect to being a reservoir for bioactive molecules, matrikines and proteases which are in a constant and dynamic balance (Figure 1); one that is likely to be much different within the aging myocardium. Unfortunately, the balance in these signaling/proteolytic pathways within the aging myocardium appears to be one that would favor a more aggressive and amplified adverse LV remodeling response following a myocardial insult.
Figure 1.
The myocardial ECM is a complex entity which forms a reservoir for bioactive signaling molecules (such as angiotensin-II, cytokines, transforming growth factor (TGF), osteopontin (OPN)), matricellular proteins which regulate ECM structure (such as secreted protein acidic and rich in cysteine;SPARC), and proteases and inhibitors (such as the matrix metalloproteinases; MMPs, and secretory-leukocyte-protease-inhibitor; SLPI). Under Normal conditions, dynamic and likely continuous interactions between prototypical receptors such as the angiotensin-II receptor to pro-fibrotic and matricellular proteins, and proteolytic enzymes determine the homeostatic balance of the ECM. The study in this issue of Circulation by Jugdutt and colleagues demonstrated that a shift in the balance if this system occurs within the aging myocardium which would likely favor a more aggressive and amplified remodeling response following an Acute MI.2 Following an acute MI, the release of cytokines such as interleukin-6 (IL-6) occurred which was associated with an induction of TGF, OPN and an array of proteolytic enzymes including MMPs (ie MMP-2, MMP-9) as well as SLPI- many of which are likely released/induced by an acute inflammatory cell response. Jugdutt and colleagues demonstrated that this cytokine/matrikine/proteolytic cascade could be modified with an infusion of an angiotensin-II receptor antagonist.
Acknowledgments
The author is grateful to Kaelyn Hawkins for creation of the figure.
Sources of Funding
This work was supported by NIH grants HL057952, HL059165 and the Research Service of the Department of Veterans Affairs.
Footnotes
Conflict of Interest Disclosures
Francis G. Spinale, MD, PhD: None
References
- 1.St John Sutton M, Pfeffer MA, Moye L, Plappert T, Rouleau JL, Lamas G, Rouleau J, Parker JO, Arnold MO, Sussex B, Braunwald E. Cardiovascular death and left ventricular remodeling two years after myocardial infarction: baseline predictors and impact of long-term use of captopril: information from the Survival and Ventricular Enlargement (SAVE) trial. Circulation. 1997;96:3294–9. doi: 10.1161/01.cir.96.10.3294. [DOI] [PubMed] [Google Scholar]
- 2.Jugdutt BI, Jelani A, Palaniyappan A, Idikio H, Uweira RE, Menon V, Jugdutt CE. 1Aging-related early changes in markers of ventricular and matrix remodeling after reperfused ST-segment elevation myocardial infarction in the canine model. Effect of early therapy with an angiotensin II type 1 receptor blocker. Circulation. 2010:948190. doi: 10.1161/CIRCULATIONAHA.110.948190. EDITORIAL OFFICE WILL PROVIDE FINAL CITATION. [DOI] [PubMed] [Google Scholar]
- 3.Spinale FG. Matrix remodeling and the matrix metalloproteinases: Influence on cardiac form and function. Physiol Rev. 2007;87:1285–342. doi: 10.1152/physrev.00012.2007. [DOI] [PubMed] [Google Scholar]
- 4.Bujak M, Kweon HJ, Chatila K, Li N, Taffet G, Frangogiannis NG. Aging-related defects are associated with adverse cardiac remodeling in a mouse model of reperfused myocardial infarction. J Am Coll Cardiol. 2008;51:1384–92. doi: 10.1016/j.jacc.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh M, Foster CR, Dalal S, Singh K. Role of osteopontin in heart failure associated with aging. Heart Fail Rev. 2010 doi: 10.1007/s10741-010-9158-6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Cao DJ, Gillette TG, Hill JA. Cardiomyocyte autophagy: remodeling, repairing, and reconstructing the heart. Curr Hypertens Rep. 2009;11:406–11. doi: 10.1007/s11906-009-0070-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Webb CS, Bonnema DD, Ahmed SH, Leonardi AH, McClure CD, Clark LL, Stroud RE, Corn WC, Finklea L, Zile MR, Spinale FG. Specific temporal profile of matrix metalloproteinase release occurs in patients following myocardial infarction: relation to left ventricular remodeling. Circulation. 2006;114:1020–7. doi: 10.1161/CIRCULATIONAHA.105.600353. [DOI] [PubMed] [Google Scholar]
- 8.Dobaczewski M, Gonzalez-Quesada C, Frangogiannis NG. The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J Mol Cell Cardiol. 2010;48:504–11. doi: 10.1016/j.yjmcc.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee Y, Gustafsson AB. Role of apoptosis in cardiovascular disease. Apoptosis. 2009;14:536–48. doi: 10.1007/s10495-008-0302-x. [DOI] [PubMed] [Google Scholar]
- 10.Dai Q, Escobar GP, Hakala KW, Lambert JM, Weintraub ST, Lindsey ML. The left ventricle proteome differentiates middle-aged and old left ventricles in mice. J Proteome Res. 2008;7:756–65. doi: 10.1021/pr700685e. [DOI] [PubMed] [Google Scholar]
- 11.Karsner HT, Dwyer JE. Studies in infarction: IV. Experimental bland infarction of the myocardium, myocardial regeneration and cicatrization. J Med Res. 1916;34:21–40.3. [PMC free article] [PubMed] [Google Scholar]
- 12.Bradshaw AD, Baicu CF, Rentz TJ, Van Laer AO, Bonnema DD, Zile MR. Age-dependent alterations in fibrillar collagen content and myocardial diastolic function: role of SPARC in post-synthetic procollagen processing. Am J Physiol Heart Circ Physiol. 2010;298:H614–22. doi: 10.1152/ajpheart.00474.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Angelov N, Moutsopoulos N, Jeong MJ, Nares S, Ashcroft G, Wahl SM. Aberrant mucosal wound repair in the absence of secretory leukocyte protease inhibitor. Thromb Haemost. 2004;92:288–97. doi: 10.1160/TH03-07-0446. [DOI] [PubMed] [Google Scholar]
- 14.Lindsey ML, Goshorn DK, Squires CE, Escobar GP, Hendrick JW, Mingoia JT, Sweterlitsch SE, Spinale FG. Age-dependent changes in myocardial matrix metalloproteinase/tissue inhibitor of metalloproteinase profiles and fibroblast function. Cardiovasc Res. 2005;66:410–9. doi: 10.1016/j.cardiores.2004.11.029. [DOI] [PubMed] [Google Scholar]