Figure 2. Halogenation and cyclopropanation in the Cur pathway.
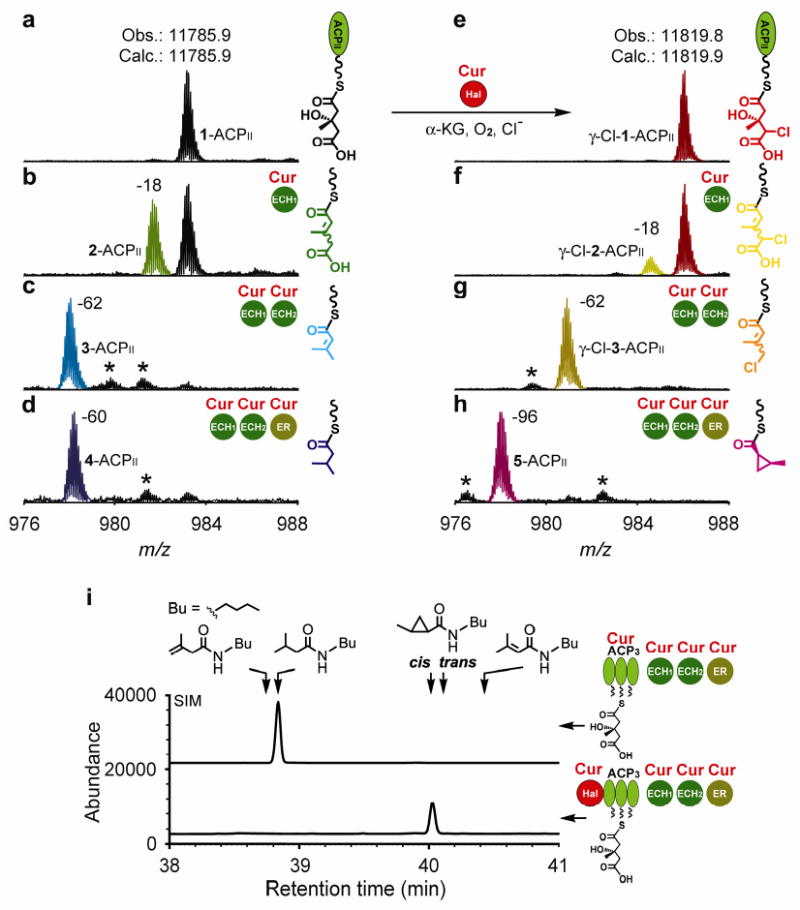
a-h, Partial FTICR mass spectra (12+ charge state of ACPII) for Cur ECH1, ECH2 and ER reactions excluding (a-d) or including (e-h) the Cur Hal chlorination step. 1-ACPII was incubated with Cur Hal for 2 h to generate the γ-Cl-1-ACPII substrate. Reactions were incubated at 30°C for 2 h for the 1-ACPII substrate and 30 min for the γ-Cl-1-ACPII substrate. Asterisks denote unidentified species. i, GC-MS analysis of the enzyme products after butylamine cleavage, and comparison with authentic standards. For optimal sensitivity, the chromatograms were recorded at selective ion mode (SIM) by monitoring 55, 57, 83, 115, 155 and 157 atomic mass unit (amu). Retention times of the products were confirmed by coinjection with the authentic standards.
