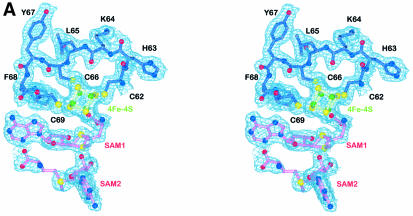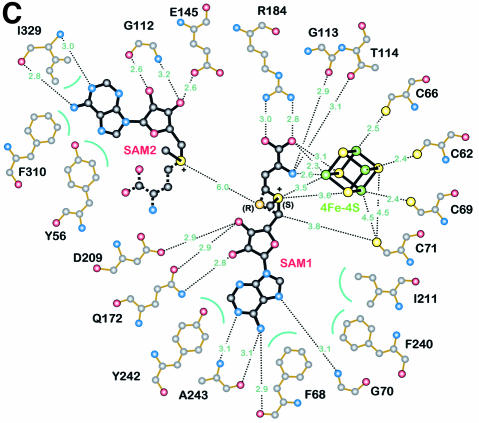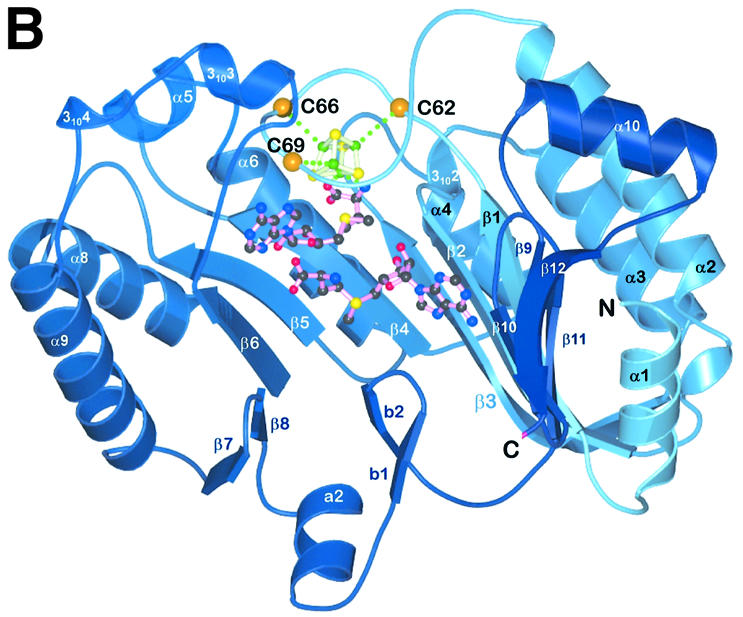
Fig. 3. Detailed views of the cofactors. (A) The electron density associated with the cofactors and the CxxxCxxC motif, conserved in all Radical SAM proteins. The 4Fe–4S cluster is rendered in green (Fe) and yellow (S), while pink-colored bonds highlight SAM1 and SAM2. Both (S)- (above) and (R)- (below) sulfonium sulfur configurations are observed for SAM1. SAM2 is rotationally disordered around the C5′–S+ bond, resulting in discontinuous electron density. (B) The cofactors occupy the central void of the catalytic domain near the C-terminal ends of the three-quarter barrel β-strands. Orange spheres mark the Cα positions of conserved cysteines. (C) A schematic depiction of inter-cofactor distances and amino acid residues involved in binding the cofactors. The (S)-sulfur is presented in yellow and the (R)-sulfur in orange. Green arcs represent hydrophobic interactions.