Abstract
Multiple myeloma (MM) cells continuously secrete large amounts of immunoglobulins that are folded in the endoplasmic reticulum (ER) whose function depend on the Ca2+ concentration inside its lumen. Recently, it was shown that the ER membrane leaks Ca2+ that is captured and delivered back by mitochondria in order to prevent its loss. Thus, we hypothesized that the highly active and abundant ER in MM cells results in greater Ca2+-regulation by mitochondria which would render them sensitive to mitochondrial inhibitors. Here, we indeed find that Ca2+ leak is greater in 3 MM, when compared to 2 B-cell leukemia cell lines. Moreover, this greater leak in MM cells is associated with hypersensitivity to various mitochondrial inhibitors, including CCCP. Consistent with our hypothesis, CCCP is more potent in inducing the unfolded protein response marker, CHOP/GADD153 in MM versus B-cell leukemia lines. Additionally, MM cells are found to be significantly more sensitive to clinically used fenofibrate and troglitazone, both of which were recently shown to have inhibitory effects on mitochondrial function. Overall, our results demonstrate that the unusually high ER activity in MM cells may be exploited for therapeutic benefit through the use of mitochondrial inhibitors including troglitazone and fenofibrate.
Keywords: Multiple myeloma, Mitochondria, Fenofibrate, Troglitazone, Calcium, Unfolded protein response
Introduction
Multiple myeloma (MM) is derived from antibody producing plasma cells and continuously secretes either antibodies or immunoglobulin light chains. To meet the demand for the large amount of immunoglobulin secretion, MM cells contain a high amount of endoplasmic reticulum (ER) as demonstrated by electron microscopic studies [20]. In the lumen of the ER, glycoproteins, including immunoglobulins, are synthesized and subsequently folded into their native confirmation prior to their transport to the Golgi apparatus. This highly complex, yet tightly regulated protein folding process, is maintained by several ER-resident proteins. All of these proteins are shown to bind Ca2+ in order to execute their function [40]. Therefore, it is not surprising that ER luminal Ca2+ concentrations are maintained at high levels in order to maintain efficient glycoprotein synthesis and folding.
The Ca2+ concentration in the ER depends on the equilibrium established by the competition between its release from the ER lumen and its import from the cytoplasm via the smooth endoplasmic reticulum ATPase (SERCA). The release of Ca2+ from the ER following induction by agonists that open the inositol triphosphate (IP3) channels or ryanodine receptors [6, 12, 33] has been well-studied. However, recently it was shown that there is a continuous ER Ca2+ leak which is independent from activation of the IP3-or ryanodine channels. This “passive leak” involves the translocon on the ER membrane, through which proteins are transported, and at the same time leaks Ca2+ following its binding to a ribosome–peptide complex [11, 45]. Although the physiological relevance of this leak is under debate, it appears that the rate of leak correlates with the number of translocons [9]. Therefore, it stands to reason that in a secretory cell like MM where there is a high rate of protein translocation from the cytoplasm into the ER, there should be greater amounts of Ca2+ leak as compared to non-secretory cells of the same lineage such as B-cell leukemias.
The greater Ca2+ leak, in turn, should render the ER function in MM cells dependent on mitochondrial activity since mitochondria are shown to play an important role in replenishing the Ca2+ content of the ER following release of this cation into the cytoplasm. As Ca2+ exits the ER, it is rapidly sequestered by mitochondria without allowing its diffusion into other compartments of the cell [1, 7]. Following its entry into the mitochondrial matrix, Ca2+ ions flow back into the ER via SERCA [29]. Thus, it follows that when mitochondria are inhibited, fluxing of Ca2+ back into the ER will be diminished. Therefore, we hypothesized that the high ER function and subsequent Ca2+ leak of MM cells will render them more susceptible to mitochondrial inhibitors, i.e. electron transport chain (ETC) blockers or uncouplers, as compared to non-antibody producing B-cells.
It has been clearly established that when ER Ca2+ is reduced, protein folding is disrupted and unfolded proteins accumulate in the ER resulting in the initiation of a complex pathway known as the unfolded protein response (UPR) [43]. Although the UPR initially upregulates genes that are involved in reestablishing ER homeostasis, it also leads to cell death if the stress in the ER is not alleviated [22]. Thus, in the present study, we investigated in several MM cell lines whether targeting mitochondria with well-known inhibitors would interfere with ER function and thereby induce UPR-mediated apoptosis.
Although the hypothesis presented here is new, exploitation of this idea for therapeutic gain is limited due to the well-characterized toxicity of the ETC. inhibitors as well as uncouplers used in this study [19, 24]. However, recently it was reported that the peroxisome proliferators-activated receptor (PPAR) agonists, i.e., troglitazone, a PPARγ agonist which is used to treat diabetes, and fenofibrate, a PPARα agonist which lowers cholesterol, uncoupled and/or inhibited mitochondrial respiration [35]. These reports prompted us to investigate whether either or both troglitazone and fenofibrate, similar to ETC inhibitors, have selective toxic activity toward MM, as compared to non-myeloma cells, and therefore may be useful in the clinic for targeting these cells.
Methods and material
Cells types
The MM cell line 8226 was purchased from American Tissue and Cell Collection (ATCC, Manassas, VA, USA) while MM.1S and KMS-11 cell lines were established as previously described [36]. B-cell leukemia lines, NALM6 and REH cells, were a kind gift from Dr. Julio Barredo from University of Miami Sylvester Comprehensive Cancer Center (Miami, FL, USA). All cell lines were grown in RPMI 1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum under 37°C and 5% CO2.
Cytotoxicity assay
Cells were incubated for 24 h at 37°C in 5% CO2 at which time drug treatments began and continued for 24 h. At this time cells were transferred to a tube followed by centrifugation at 400g for 5 min. The pellets were resuspended in 1 ml of Hanks solution and analyzed by Vi-Cell (Beckman Coulter, Fullerton, CA, USA) cell viability analyzer.
Assaying mitochondrial function
Two parameters were assayed for mitochondrial function: Δψm and oxygen consumption. Δψm was estimated using 5,5′,6,6′-tetraethylbenzimidazole carbocyanide iodide (JC-1, Invitrogen, Carlsbad, CA, USA) as described in [2]. Oxygen consumption was measured in 3 × 106 cells using a Clark’s electrode (Hansatech, Cambridge, UK) as described previously [25].
Measurement of cytoplasmic and mitochondrial calcium
Cytoplasmic Ca2+ concentration was estimated by using the cell permeant ratiometric fluorochrome indo-1-AM (Invitrogen, Carlsbad, CA, USA) while mitochondrial Ca2+ was measured by X-Rhod-1-AM (Invitrogen, Carlsbad, CA, USA). In the latter assay, to normalize mitochondrial Ca2+ signal to mitochondria number, Mitotracker Green (Invitrogen, Carlsbd, CA, USA) fluorescence was simultaneously analyzed. Experiments were performed in cells loaded with either 2.5 μM of indo-1 or 5 μM X-rhod-1 and 250 nM of Mitotracker at 37°C for 45 min. Cells were, then, centrifuged at 400g for 5 min and resuspended in their growth medium followed by distribution of 100 μl of aliquots into 96 well optical bottom plates (Nalge Nunc, Int., Rochester, NY, USA) and fluorescence was measured by Spectra Max Gemini Plus (Molecular Devices, Sunnyvale, CA, USA). The average of triplicates from untreated samples was used as control reading and increase in cytoplasmic or mitochondrial Ca2+ was calculated as percent increase from control samples.
Western blot analysis
Western blots were performed as described previously [25]. Membranes were probed with monoclonal rabbit anti-GRP94, anti GRP-78, anti-PDI, anti-CHOP/GADD153, anti-cleaved caspase 3 (Cell Signaling, Danvers, MA, USA) and monoclonal mouse anti-β-actin (Sigma, St. Louis, MO, USA).
Results
The ER of MM cells leak more Ca2+ than the ER of B-cell leukemias
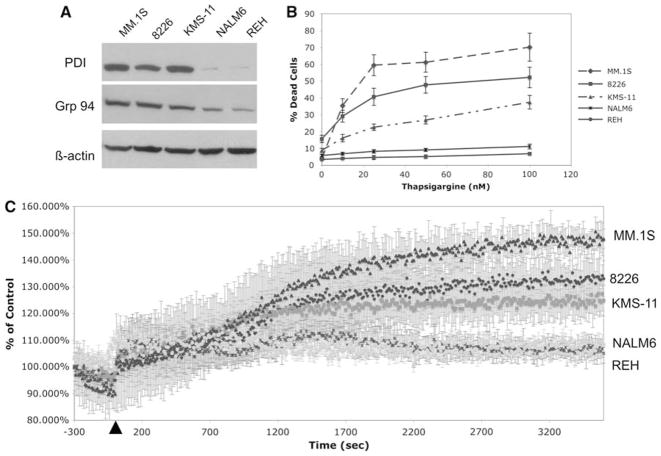
As shown in Fig. 1a, MM cell lines (MM.1S, 8226, KMS-11), as compared to B-cell leukemias (NALM6 and REH), express significantly greater amounts of ER-resident proteins, i.e., glucose-regulated protein 94 (GRP94), protein disulfide isomerase (PDI), which correlates with their highly upregulated secretory function. Since Ca2+ is required for the enzymatic activity of most of the ER-resident proteins, its concentration in the ER is important to ensure correct folding and thereby avoid ER stress. Thus, it could be expected that MM cells would be particularly sensitive to changes in ER Ca2+. To investigate this possibility, we assayed the toxicity of thapsigargine, an inhibitor of the main ER Ca2+ pump, SERCA. Indeed, when treated with this agent, all 3 MM cell lines are found to be sensitive to it while at equivalent doses 2 B-cell leukemias are resistant (Fig. 1b).
Fig. 1.
Higher expression of ER-resident proteins correlates with hypersensitivity to thapsigargine and increased ER Ca2+ leak in MM as compared to non-myeloma cell lines. a Expression of two ERresident proteins, GRP94 and PDI, was assayed by western blot in all 5 cell lines. b Sensitivity to the SERCA inhibitor, thapsigargine, in MM cell lines, as assayed by trypan blue exclusion assays following 24 h treatment. The data is an average of triplicate samples from one of three experiments. c ER Ca2+ leak was estimated by the increase in the ratio of indo-1 fluorescence emitted at 400 versus 500 nm following thapsigargine treatment. Thapsigargine was added after 5 min of basal calcium measurement. The data represents the percent increase of the ratio of indo-1 fluorescence as compared to control levels and the average + SD of triplicate samples
Recently, the transport of glycoproteins from cytoplasm into the ER has been shown to be associated with Ca2+ leak. Due to the high levels of glycoprotein production in MM cells, we investigated whether there is a greater ER Ca2+ leak in these cells as compared to B-cells. As shown in previous reports, ER Ca2+ leak can be assayed indirectly by measuring cytoplasmic Ca2+ concentration following inhibition of SERCA by thapsigargine [38, 45]. Within 5 min after addition of thapsigargine, cytoplasmic Ca2+ concentration significantly increases in all five cell lines. However, after 30 min cytoplasmic Ca2+ concentration stabilizes at its new equilibrium in which all 3 MM cell lines had significantly greater cytoplasmic Ca2+ concentration than the 2 B-cell leukemias indicating that ER Ca2+ leak is greater in the former cell type. Furthermore, the order of ER Ca2+ leak is found to be MM1.S > 8226 > KMS-11 which correlates with the order of sensitivity to thapsigargine (Fig. 1c). Taken together, these findings indicate that MM cells have a more profound Ca2+ leakage from their ER membrane than B-cell leukemias which correlates with their greater sensitivity to SERCA inhibition.
Increased ER Ca2+ leak in MM cells is associated with hypersensitivity to mitochondrial inhibitors
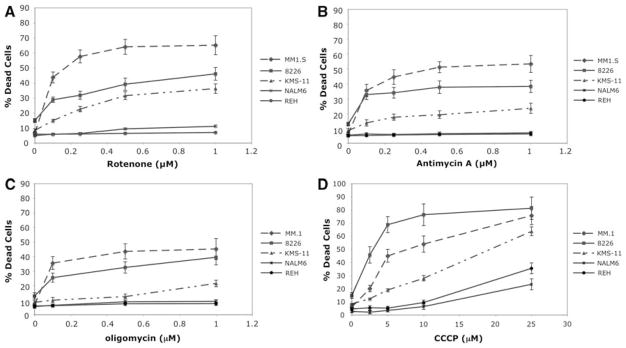
Since mitochondria play an important role in replenishing ER Ca2+ content following its exit from ER lumen, we were prompted to investigate whether MM cells are more sensitive to mitochondrial inhibitors due to their greater ER Ca2+ leak. Here, we demonstrate that all 3 MM cell lines undergo significant cell death following 24 h treatment with rotenone (complex I inhibitor), antimycin A (complex III inhibitor) and oligomycin (complex V inhibitor) at concentrations that induce little or no toxicity in the 2 B-cell leukemia lines (Fig. 2a–c). A common outcome of 24 h treatment with these distinct mitochondrial inhibitors is the reduction in Δψm. This potential is reported to be a major factor for capturing cytoplasmic Ca2+ and re-directing it back to the ER thereby preventing reduction of ER Ca2+ content.
Fig. 2.
MM cell lines are more sensitive to mitochondrial inhibitors as compared to B-cell leukemia. Cytotoxicity was measured by trypan blue exclusion assays following 24 h treatment with (a) rotenone, (b) antimycin A, (c) oligomycin and (d) CCCP in five cell lines. The data is an average of triplicate samples + SD from one of three experiments
To more directly demonstrate that reduction of Δψm is the underlying mechanism for hypersensitivity of MM cells to mitochondrial inhibitors we used a well-known uncoupler, CCCP, that directly dissipates mitochondrial proton gradient. Similar to other mitochondrial inhibitors, CCCP is also more toxic to MM cells as compared to non-myeloma cells (Fig. 2d). It is important to note that CCCP is known to be a classical inducer of apoptosis [23] and therefore with higher concentrations of this agent, significant toxicity is induced in all cell types. However, the concentration of CCCP as well as other OxPhos inhibitors required to induce cell death in all 3 MM cells is significantly less than that required for B-cell leukemias.
All OxPhos inhibitors used in these studies display a similar pattern of potency where the order of sensitivity is found to be MM.1S > 8226 > KMS-11. This pattern is consistent with the rate of ER Ca2+ leak in these cells (Fig. 1c) which suggests that the mechanism of cell death induced by mitochondrial inhibitors may be related to their ability to perturb mitochondria–ER Ca2+ recycling.
Greater sensitivity of MM cells, as compared to B-cell leukemias, to mitochondrial inhibitors does not correlate with intrinsic differences in their mitochondrial function
To rule out the possibility that heightened sensitivity of MM cells to reduction in Δψm results from deficiencies in the mitochondrial function of these cells as compared to B-cell leukemias, we compared the oxygen consumption as well as Δψm in all five cell lines. All MM cells appear to respire 40–50% more than 2 B-cell leukemias indicating that deficiency in the mitochondrial activity of the former cells is not a likely reason for their hypersensitivity to mitochondrial inhibitors (Table 1). Similarly, when Δψm is estimated by the ratio of aggregated versus non-aggregated JC-1 dye, inherent differences in Δψm between these cell lines are found not to correlate with their differential sensitivity to mitochondrial inhibitors. Therefore, intrinsic differences in mitochondrial function does not appear to be the major determinant for hypersensitivity of MM cells to mitochondrial inhibitors.
Table 1.
Comparison of mitochondrial function between MM and B-cell leukemias demonstrate that intrinsic differences in the mitochondrial activity does not account for the hypersensitivity of the MM cell lines to mitochondrial inhibitors
| Cell lines | Oxygen consumption (nmol/106 cells/min) |
Δψm (ratio of JC-1 red/green) | |
|---|---|---|---|
| Basal | After 2 mM of KCN | ||
| MM.1S | 1.43 ± 0.13 | 0.15 ± 0.0096 | 9.635 |
| 8226 | 1.52 ± 0.19 | 0.1 ± 0.0087 | 1.367 |
| KMS-11 | 1.53 ± 0.2 | 0.137 ± 0.011 | 4.678 |
| NALM6 | 0.95 ± 0.11 | 0.089 ± 0.0078 | 9.985 |
| REH | 1.12 ± 0.12 | 0.1 ± 0.0093 | 9.467 |
CCCP has similar effects on Δψm and mitochondrial Ca2+ uptake in MM versus B-cell leukemic cell lines
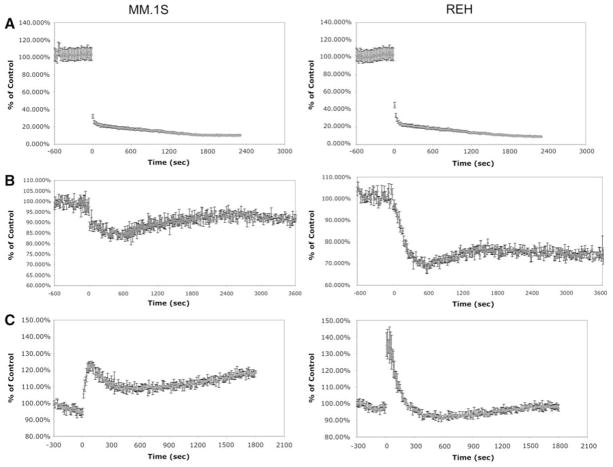
To investigate the underlying factor for heightened sensitivity of MM cells to mitochondrial inhibitors we used CCCP, since the oxidative stress generated by the other inhibitors can alter Ca2+ homeostasis directly which could complicate the interpretation of the results. When the effects of CCCP were compared in the MM.1S versus REH cell lines, it was determined that immediately after addition of 10 μM of CCCP, Δψm is significantly reduced in both cell types (Fig. 3a). Moreover, this reduction in Δψm coincides with lowering of Ca2+ concentration in the mitochondrial matrix while cytoplasmic Ca2+ levels go up in both cell types (Fig. 3b, c). These data indicate that perturbation of mitochondrial proton gradient leads to release of free Ca2+ ions from the matrix of this organelle into the cytoplasm in both cell types. Furthermore, 5–10 min following their entry into the cytoplasm, Ca2+ ions appear to be cleared from this compartment as can be seen by the rapid reduction of cytoplasmic Ca2+ concentration in both cell lines (Fig. 3). However, it is important to note that cytoplasmic Ca2+ levels go back to control levels in the B-cell leukemia, REH, while they remain elevated in the MM cell line, MM.1S. This sustained increase in cytoplasmic Ca2+ concentration of MM1.S cell line may be explained by the greater continuous leak of this cation from the ER of MM.1S, which cannot be captured by their mitochondria under these conditions (Fig. 1c). Taken together, these findings suggest that CCCP has similar effects on the mitochondria of both MM and B-cell leukemia lines and does not account for the hypersensitivity of MM cells to mitochondrial inhibition.
Fig. 3.
CCCP has similar effects on ψm potential, cytoplasmic Ca2+ and mitochondrial Ca2+ of MM.1S and REH cells. a ψm potential, b mitochondrial Ca2+ and c cytoplasmic Ca2+ levels were measured using the fluorochromes JC-1, X-rhod-1 and indo-1, respectively. After 10 min of initial baseline measurement, CCCP was added into each well to achieve a final concentration of 10 μM and changes in fluorescence intensity was assayed for up to 2 h. In the graphs the time point of CCCP addition is marked as ‘0’. The graph is an average of triplicate samples with + SD
Induction of unfolded protein response is associated with cell death induced by CCCP in MM cells
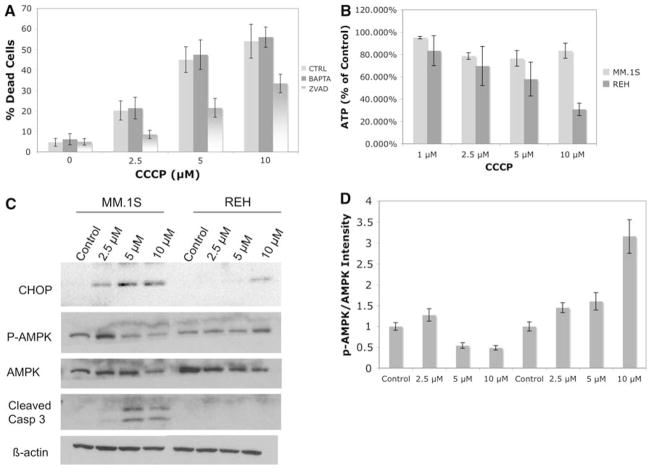
As demonstrated above, treatment of MM cells with CCCP drastically reduces Δψm and mitochondrial Ca2+ content while increasing its cytoplasmic levels. To investigate how these events lead to cell death in MM cells, we focused on three possibilities: (i) Ca2+-mediated toxicity as a result of accumulation of this cation in the cytoplasm of MM cells: (ii) inhibition of ATP synthesis following dissipation of the proton gradient by CCCP: (iii) induction of UPR due to perturbation of mitochondrial Ca2+ loading of ER. The first possibility is unlikely for the following reasons: (1) Addition of the membrane permeable Ca2+ chelator, BAPTA-AM does not reverse the toxicity of CCCP in MM.1S cells (Fig. 4a); (2) It appears from the literature that cell death due to cytosolic Ca2+ accumulation occurs via activation of numerous cytotoxic pathways including calpains, endonucleases and caspases while classical mitochondrial apoptosis is primarily caspase-dependent [21]. Based on these reports, we tested and found that the pan-caspase inhibitor Z-VAD can reverse the majority of CCCP toxicity in MM.1S cells suggesting that cell death induced by this agent is mediated mainly by the classical mitochondrial apoptotic pathway and not due to accumulation of Ca2+ in the cytoplasm (Fig. 4a).
Fig. 4.
CCCP induced cell death in MM appears to be mediated by UPR. a Reversal of CCCP induced toxicity by either 10 μM BAPTA-AM or 50 μM of Z-VAD was investigated using trypan blue exclusion assays. The bars represent the average of triplicate samples + SD. b ATP levels were assayed following 6 h of treatment with indicated concentrations of CCCP. The bars represent the average of triplicate samples + SD. c Induction of UPR, activation of AMPK pathway and cleavage of caspase 3 was analyzed by western blots following 24 h treatment with indicated concentrations of CCCP in MM.1S and REH cell lines. β-Actin was used as a loading control. d The quantification of phosphorylated AMPK and total AMPK bands in the previous panel was done using a Bio-Rad gel reader which employs Quality I software. The bars represent the fold increase in the ratio of p-AMPK/AMPK in treated versus untreated samples from three independent experiments + SD
As mentioned above, a possible explanation of why CCCP is more potent in inducing apoptosis in MM.1S versus REH cells is that the former cell type may be more susceptible to ATP depletion by this treatment. However, as demonstrated in Fig. 4b, ATP levels are decreased more significantly in REH cells, as compared to MM.1S cells. Furthermore, consistent with greater reduction of ATP in REH cells following CCCP treatment, the cytoplasmic ATP sensor, AMPK, is found to be more phosphorylated in these cells at the highest dose (10 μM) (Fig. 4c). At the lowest dose (2.5 μM), when the ratio of phosphorylated versus non-phosphorylated AMPK bands are measured by densitometry (Fig. 4d), a similar increase is found in both cell types which correlates with their similar reductions in ATP levels (Fig. 4b). At higher doses, AMPK phosphorylation is suppressed in MM.1S cells while it continues to increase in REH cells (Fig. 4d). Overall, these data indicate that ATP depletion resulting from CCCP treatment does not appear to be the underlying reason for the heightened sensitivity of MM cells to this agent.
A third possibility is offered by the intricate relationship between mitochondria and ER for replenishing Ca2+ in the latter organelle. Above, we demonstrated that the ER of MM cells leak significantly more Ca2+ than B-cell leukemias and thus it follows that upon inhibition of mitochondrial Ca2+ uptake by CCCP, the ER Ca2+ concentrations will decrease more abruptly in MM cells as compared to B-cell leukemias. Since we were not able to measure ER Ca2+ directly, we assayed induction of UPR as a marker of reduced ER Ca2+ concentration. It is well-known that interference with ER Ca2+ levels leads to initiation of UPR, which if severe enough or prolonged, results in cell death [12, 43]. Among various markers of UPR, we selected those from the PERK pathway, i.e. CHOP/GADD153, since the two other ER stress signal transducers, IRE1 and ATF6, are shown to be constitutively active in order to maintain the high ER function of MM cells [18, 36]. Following treatment with 2.5 μM of CCCP, there is significant induction of CHOP/GADD153 expression in MM.1S cells while at least 10 μM of this agent is required to cause a similar increase in REH cells (Fig. 4c). Furthermore, consistent with greater toxicity of CCCP in MM cells, the major executioner caspase, caspase 3, starts to get cleaved following treatment with 5 μM of CCCP while no cleaved caspase 3 can be detected in REH cells even at the 10 μM dose. Interestingly, the upstream markers of the PERK pathway, i.e., eif2α phosphorylation and ATF4 upregulation, were not detected in MM.1S cells treated with CCCP (data not shown). Although the induction of CHOP/GADD153 is generally known as a reliable marker of the PERK pathway, another possibility comes from recent reports in which CHOP/GADD153 up-regulation is shown to also occur as a result of mitochondrial UPR which is independent from the PERK pathway [17]. However, this possibility remains questionable since transducers of the mitochondrial UPR in mammalian cells are currently unknown. Overall, these results suggest that the apoptotic cell death induced by dissipation of Δψm in MM results mainly from perturbation of protein folding either in the ER or mitochondria of these cells and not from ATP depletion or cytosolic Ca2+ accumulation.
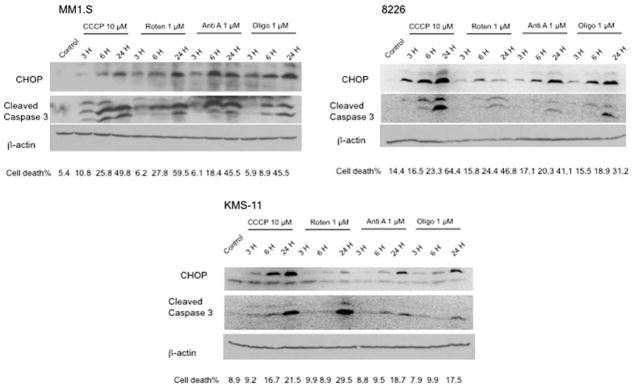
Mitochondrial inhibitors induce UPR in all 3 MM cell lines
In addition to CCCP, when MM cells are treated with other inhibitors of mitochondria, i.e. rotenone, antimycin A and oligomycin, for various time points, CHOP/GADD153 induction can also be detected. Three hours of incubation with all 4 mitochondrial inhibitors resulted in increased expression of CHOP/GADD153 in all 3 MM cell lines (Fig. 5). When the effects of CCCP are compared in 3 cell lines, the induction of CHOP/GADD153 appears to be more profound in MM.1S and 8226 as compared to KMS-11 which correlates with the less toxic effects of this agent in the latter cell line (Fig. 5). Similarly, caspase 3 cleavage occurs more rapidly in MM.1S and 8226 versus KMS-11 cells following CCCP treatment.
Fig. 5.
All mitochondrial inhibitors induce CHOP expression in 3 MM cell lines. Induction of UPR-mediated apoptosis following treatment of 3 MM cell lines with four different mitochondrial inhibitors for various time points was assayed by western blot analysis of CHOP and cleaved caspase 3. Simultaneously, cytotoxicity was measured by trypan blue exclusion assays and percentage of cell death is demonstrated below each sample
When ETC inhibitors were tested, rotenone induced less CHOP/GADD153 expression than CCCP although at the concentrations used in these experiments they both resulted in a similar magnitude of cell death (Fig. 5). Moreover, both antimycin A and oligomycin treatments lead to increased expression of CHOP/GADD153 comparable to that induced by CCCP although CCCP is more toxic than either antimycin A or oligomycin as demonstrated by caspase 3 cleavage and percent of cell death (Fig. 5). This finding indicates that effects other than perturbation of protein folding may also be contributing to the toxicity of mitochondrial inhibitors in MM cells.
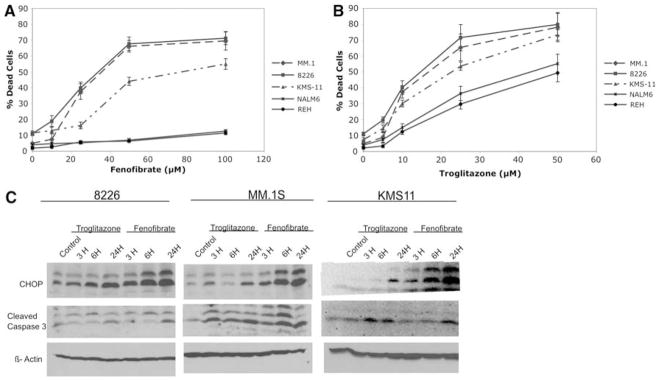
PPAR agonists troglitazone and fenofibrate mimic mitochondrial inhibitors in inducing CHOP/GADD153 and cell death in MM cell lines
Recently, it was reported that agonists of both PPARα and PPARγ, fenofibrate and troglitazone, respectively, inhibit mitochondrial respiration at various complexes which may be in part responsible for their clinically beneficiary effects of lowering of lipids or glucose [35], respectively. Since we find that inhibition of mitochondria is associated with UPR induction and apoptosis in MM cell lines, we tested whether treatment with either fenofibrate or troglitazone results in cell death in a manner similar to mitochondrial inhibitors. Both of these agents induce significant toxicity in all 3 MM cell lines at doses that are less toxic to B-cell leukemia lines (Fig. 6a, b). Interestingly, troglitazone, when compared to fenofibrate, is found to be more toxic to B-cell leukemias. Therefore, fenofibrate may have more direct effects on ER-mitochondria Ca2+ coupling while troglitazone may be interfering with other cellular processes in addition to its effects on ER function. This contention is further supported when CHOP/GADD153 induction by either of these agents is assayed. As shown in Fig. 6c, fenofibrate treatment leads to higher expression of CHOP/GADD153 than troglitazone, although at the concentrations used in this experiment they both result in similar levels of cytotoxicity. Thus, both fenofibrate and troglitazone appear to mimic other mitochondrial inhibitors in their ability to target MM cells via either ER or mitochondrial UPR although factors other than interference with protein folding may also be contributing to troglitazone mediated toxicity.
Fig. 6.
Troglitazone and fenofibrate are similar to mitochondrial inhibitors in inducing more toxicity in MM versus B-cell leukemia lines which associates with increases in CHOP/GADD153 expression. Cytotoxicity was measured by trypan blue exclusion assays following 24 h of treatment with either a fenofibrate or b troglitazone. The graph demonstrates the average of triplicate samples + SD. c Induction of UPR-mediated apoptosis by troglitazone and fenofibrate was assayed by western blot analysis of CHOP/GADD153 and cleaved caspase 3 levels
Discussion
In several reports, it has been demonstrated that mitochondria are important in maintaining ER Ca2+ concentrations following its release from the ER [1, 7, 12, 16, 26, 29, 30]. When ER Ca2+ levels decrease, mitochondria sequester this cation from the cytoplasm and subsequently transport it back into the ER via SERCA. This relationship between the mitochondria and the ER in regulating Ca2+, also known as store-operated Ca2+ entry (SOCE), is well-studied in cells that release Ca2+ from the ER following induction by agonists that open inositol triphosphate (IP3) channels or ryanodine receptors [6, 12, 33]. However, it has recently been shown that Ca2+ can also be released from the ER without activation of the IP3- or ryanodine channels. This release involves the translocon on the ER membrane, through which proteins are transported and leaks Ca2+ following the binding of the translocon to a ribosome–peptide complex [11, 45]. Thus, it appears that there is a correlation between the rate of protein transport through the ER membrane and the leaking of Ca2+ from this organelle. This finding suggests that a cell that synthesizes unusually high levels of secretory proteins such as MM, would leak more Ca2+ from its ER and thus be hypersensitive to Ca2+ deprivation. Indeed, we find that treatment with thapsigargine, an agent that blocks entrance of Ca2+ into the ER thru SERCA, results in greater cytotoxicity in MM as compared to B-cell leukemias cell lines (Fig. 1b). Additionally, cell death was preceded by a higher increase in cytoplasmic Ca2+ concentration of MM cells (Fig. 1c). These data suggest that SOCE is more active in MM cells as compared to B-cell leukemias and therefore may underlie the heightened sensitivity of MM to agents that perturb ER Ca2+ either directly (by thapsigargine) or indirectly (by mitochondrial inhibitors).
There is a great deal of evidence to demonstrate how Δψm plays an important role in the uptake of Ca2+ by mitochondria [3, 13, 14, 41]. However, the exact role of how each ETC complex contributes to the uptake and extrusion of mitochondrial Ca2+ has yet to be resolved. Therefore, we selected CCCP, which directly reduces Δψm, to investigate the possible reasons for the heightened sensitivity of MM cells we find in response to the reduction in mitochondrial proton gradient. Immediately after addition of CCCP, Δψm was dissipated and subsequently mitochondrial Ca2+ was extruded into the cytoplasm of both MM and B-cell leukemic cell lines. Thus, our results demonstrate that lowering of Δψm by CCCP renders mitochondria incapable of retaining Ca2+ ions in both B-cell leukemia and MM cells, and therefore the differential response of each cell type to this activity of CCCP can not account for the increased sensitivity of MM to mitochondrial inhibition.
To determine whether, inhibition of mitochondrial Ca2+ uptake generates greater ER stress in MM versus B-cell leukemia, we assayed CHOP/GADD153 expression. Although CHOP/GADD153 expression was initially identified as a marker for DNA damage and/or oxidative stress [10, 15, 37, 46, 47], subsequent reports suggest that induction of CHOP/GADD153 is more responsive to ER stress than to either DNA damage or oxidative stress [27, 34, 44]. Figure 4c shows that CCCP is four times more potent in upregulating CHOP/GADD153 in MM than in B-cell leukemia. On the other hand, when upstream markers of the PERK pathway, including eif2α phosphrylation and ATF4 expression, were assayed at various time points following treatment with mitochondrial inhibitors, their upregulation was not observed. It appears that detection of phosphorylated PERK in MM cells appears to present a challenge since PERK and thereby eiF2α phosphorylation is rapidly turned off in these cells to maintain continuous synthesis of immunoglobulins [42]. Another possibility to explain why upstream markers of PERK can not be detected in MM cells treated with mitochondrial inhibitors is based on recent studies demonstrating that perturbation of protein folding in the mitochondrial matrix can also lead to CHOP/GADD153 induction independently from the PERK pathway [17]. Thus, it can be hypothesized that the high ER activity in MM cells renders their mitochondria susceptible to interference with protein folding in the matrix of this organelle. Further understanding of the mitochondrial UPR could facilitate testing of this novel idea. Nevertheless, our findings that CCCP is more potent in inducing CHOP/GADD153 expression in MM versus B-cell leukemia indicate that inhibition of mitochondrial function results in greater perturbation of protein folding either in the ER and/or mitochondria of the former cell type which correlates with greater cell death in these cells.
Similar to CCCP, all of the other mitochondrial inhibitors tested, also resulted in increased CHOP/GADD153 expression although the level of induction was found to be different for each inhibitor. This finding could be explained by the differential ability of each ETC inhibitor to reduce Δψm, which is dependent on what fraction of the proton gradient is regulated by the complex that each agent interacts with. Furthermore, although there is a great deal of evidence to show that Δψm plays a role in mitochondrial Ca2+ uptake, the contribution of each ETC complex to mitochondrial Ca2+ uptake remains to be investigated. Thus, although our results clearly demonstrate that repression of mitochondrial function results in ER stress and thereby UPR activation in MM cell, further studies are required to determine how each inhibitor affects the mitochondria–ER relationship.
When ER stress is not alleviated, apoptosis ensues and CHOP/GADD153 expression has been associated with this UPR-mediated mode of cell death [28]. Various mechanisms are offered to explain how CHOP/GADD153 may initiate apoptosis such as suppression of bcl-2; reinitiating protein translation in an already overwhelmed ER and transfer of Ca2+ from ER to mitochondria which results in Ca2+-mediated toxicity [39]. Nevertheless, the major outcome of sustained ER stress appears to be initiation of classical apoptotic pathways mediated by caspases [39]. Here, we demonstrate that the pancaspase inhibitor Z-VAD could reverse CCCP toxicity in MM which supports the hypothesis that reduction in Δψm induces cell death via apoptosis. However, Z-VAD is a pancaspase inhibitor and therefore from this data we cannot determine which caspases play the major role in induction of apoptosis in MM cells treated with mitochondrial inhibitors. Since all of these agents have various effects on mitochondrial function, it is possible that different caspases may be activated depending on the mitochondrial inhibitor. On the other hand, Ca2+ chelation could not alter CCCP toxicity in MM cells, indicating that cytoplasmic Ca2+ accumulation due to mitochondrial inhibition does not appear to play a major role in induction of apoptosis. Thus, although further experiments may be required to better understand how ER stress is involved in mitochondrial inhibitor induced toxicity in MM cells, our results demonstrate that dissipation of Δψm in these cells associates with an UPR-mediated apoptosis.
Recently, it has been demonstrated that the clinically utilized PPAR agonists fenofibrate and troglitazone, which induce PPARα and PPARγ, respectively, have anti-mitochondrial activity [4, 5, 8, 31, 32, 35]. These results prompted the use of these drugs in our studies. Similar to what we found for the mitochondrial agents, fenofibrate and troglitazone were shown to be preferentially toxic in MM versus B-cell lines (Fig. 6). Furthermore, both of these agents induce CHOP/GADD153 expression indicating the mechanism of cell death is also associated with UPR. Previously, it was reported that troglitazone is toxic to MM cells although the mechanism was not offered. On the other hand, it is important to note that troglitazone was more toxic than fenofibrate to B-cell leukemias indicating that it interferes with cellular pathways other than ER-mitochondria Ca2+ exchange while fenofibrate appears to be more specifically targeting this latter process. Our data indicate that fenofibrate and to a lesser degree troglitazone, (via their anti-mitochondrial effects) may prove to be a new way to treat MM.
Based on the highly upregulated ER function of MM cells our findings appear to reveal an Achilles’ heel in this disease that may be exploitable with mitochondrial agents. Furthermore, other diseases in which enhanced ER function plays a role in their pathogenicity, may also prove to be similarly vulnerable to mitochondrial agents.
Acknowledgments
This work is supported by the NCI grant# CA37109 awarded to T.J.L.
Abbreviations
- CCCP
Carbonyl cyanide m-chlorophenylhydrazone
- CHOP/GADD153
C/EBP homologous protein/DNA damage-inducible gene 153
- ER
Endoplasmic reticulum
- ETC
Electron transport chain
- GRP
Glucose-regulated protein
- PDI
Protein disulfide isomerase
- PERK
PKR-like ER kinase
- PPAR
Peroxisome-proliferator activated receptor
- MM
Multiple myeloma
- SERCA
Smooth endoplasmic reticulum Ca2+-ATPase
- UPR
Unfolded protein response
- Δψm
Mitochondrial membrane potential
Footnotes
Conflict of interest statement None.
Contributor Information
Metin Kurtoglu, Department of Cell Biology and Anatomy and Sylvester Comprehensive Cancer Center, University of Miami Miller School of Medicine, 1550 NW 10th Ave, PAP Bldg, Room# 115, Miami, FL 33136, USA.
Katherine Philips, Department of Cell Biology and Anatomy and Sylvester Comprehensive Cancer Center, University of Miami Miller School of Medicine, 1550 NW 10th Ave, PAP Bldg, Room# 115, Miami, FL 33136, USA.
Huaping Liu, Department of Cell Biology and Anatomy and Sylvester Comprehensive Cancer Center, University of Miami Miller School of Medicine, 1550 NW 10th Ave, PAP Bldg, Room# 115, Miami, FL 33136, USA.
Lawrence H. Boise, Department of Microbiology and Immunology and Sylvester Comprehensive Cancer Center, University of Miami Miller School of Medicine, Miami, FL 33136, USA
Theodore J. Lampidis, Email: tlampidi@med.miami.edu, Department of Cell Biology and Anatomy and Sylvester Comprehensive Cancer Center, University of Miami Miller School of Medicine, 1550 NW 10th Ave, PAP Bldg, Room# 115, Miami, FL 33136, USA
References
- 1.Arnaudeau S, Kelley WL, Walsh JV, Jr, Demaurex N. Mitochondria recycle Ca(2+) to the endoplasmic reticulum and prevent the depletion of neighboring endoplasmic reticulum regions. J Biol Chem. 2001;276:29430–29439. doi: 10.1074/jbc.M103274200. [DOI] [PubMed] [Google Scholar]
- 2.Barrientos A, Moraes CT. Titrating the effects of mitochondrial complex I impairment in the cell physiology. J Biol Chem. 1999;274:16188–16197. doi: 10.1074/jbc.274.23.16188. [DOI] [PubMed] [Google Scholar]
- 3.Bianchi K, Rimessi A, Prandini A, Szabadkai G, Rizzuto R. Calcium and mitochondria: mechanisms and functions of a troubled relationship. Biochim Biophys Acta. 2004;1742:119–131. doi: 10.1016/j.bbamcr.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 4.Brunmair B, Lest A, Staniek K, Gras F, Scharf N, Roden M, Nohl H, Waldhausl W, Furnsinn C. Fenofibrate impairs rat mitochondrial function by inhibition of respiratory complex I. J Pharmacol Exp Ther. 2004;311:109–114. doi: 10.1124/jpet.104.068312. [DOI] [PubMed] [Google Scholar]
- 5.Brunmair B, Staniek K, Gras F, Scharf N, Althaym A, Clara R, Roden M, Gnaiger E, Nohl H, Waldhausl W, Furnsinn C. Thiazolidinediones, like metformin, inhibit respiratory complex I: a common mechanism contributing to their antidiabetic actions? Diabetes. 2004;53:1052–1059. doi: 10.2337/diabetes.53.4.1052. [DOI] [PubMed] [Google Scholar]
- 6.Carafoli E. Intracellular calcium homeostasis. Annu Rev Biochem. 1987;56:395–433. doi: 10.1146/annurev.bi.56.070187.002143. [DOI] [PubMed] [Google Scholar]
- 7.Csordas G, Thomas AP, Hajnoczky G. Quasi-synaptic calcium signal transmission between endoplasmic reticulum and mitochondria. EMBO J. 1999;18:96–108. doi: 10.1093/emboj/18.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feinstein DL, Spagnolo A, Akar C, Weinberg G, Murphy P, Gavrilyuk V, Dello Russo C. Receptor-independent actions of PPAR thiazolidinedione agonists: is mitochondrial function the key? Biochem Pharmacol. 2005;70:177–188. doi: 10.1016/j.bcp.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 9.Flourakis M, Van Coppenolle F, Lehen’kyi V, Beck B, Skryma R, Prevarskaya N. Passive calcium leak via translocon is a first step for iPLA2-pathway regulated store operated channels activation. FASEB J. 2006;20:1215–1217. doi: 10.1096/fj.05-5254fje. [DOI] [PubMed] [Google Scholar]
- 10.Fornace AJ, Jr, Alamo I, Jr, Hollander MC. DNA damage-inducible transcripts in mammalian cells. Proc Natl Acad Sci USA. 1988;85:8800–8804. doi: 10.1073/pnas.85.23.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giunti R, Gamberucci A, Fulceri R, Banhegyi G, Benedetti A. Both translocon and a cation channel are involved in the passive Ca2+ leak from the endoplasmic reticulum: a mechanistic study on rat liver microsomes. Arch Biochem Biophys. 2007;462:115–121. doi: 10.1016/j.abb.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 12.Gorlach A, Klappa P, Kietzmann T. The endoplasmic reticulum: folding, calcium homeostasis, signaling, and redox control. Antioxid Redox Signal. 2006;8:1391–1418. doi: 10.1089/ars.2006.8.1391. [DOI] [PubMed] [Google Scholar]
- 13.Graier WF, Frieden M, Malli R. Mitochondria and Ca(2+) signaling: old guests, new functions. Pflugers Arch. 2007;455:375–396. doi: 10.1007/s00424-007-0296-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hajnoczky G, Csordas G, Das S, Garcia-Perez C, Saotome M, Sinha Roy S, Yi M. Mitochondrial calcium signalling and cell death: approaches for assessing the role of mitochondrial Ca2+ uptake in apoptosis. Cell Calcium. 2006;40:553–560. doi: 10.1016/j.ceca.2006.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He S, Yaung J, Kim YH, Barron E, Ryan SJ, Hinton DR. Endoplasmic reticulum stress induced by oxidative stress in retinal pigment epithelial cells. Graefes Arch Clin Exp Ophthalmol. 2008;246:677–683. doi: 10.1007/s00417-008-0770-2. [DOI] [PubMed] [Google Scholar]
- 16.Hofer AM, Landolfi B, Debellis L, Pozzan T, Curci S. Free [Ca2+] dynamics measured in agonist-sensitive stores of single living intact cells: a new look at the refilling process. EMBO J. 1998;17:1986–1995. doi: 10.1093/emboj/17.7.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horibe T, Hoogenraad NJ. The chop gene contains an element for the positive regulation of the mitochondrial unfolded protein response. PLoS One. 2007;2:e835. doi: 10.1371/journal.pone.0000835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang H, Gao W, Sze DM, Xiong H, Hou J. Transcription factors Xbp-1, Blimp-1, and BSAP are involved in the regulation of plasmacytic differentiation induced by 2-methoxyestradiol in myeloma cell lines. Int J Hematol. 2007;86:429–437. doi: 10.1007/BF02984001. [DOI] [PubMed] [Google Scholar]
- 19.Jones DC, Miller GW. The effects of environmental neurotoxicants on the dopaminergic system: a possible role in drug addiction. Biochem Pharmacol. 2008;76:569–581. doi: 10.1016/j.bcp.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 20.Karpas A, Harder L, Czepulkowski B, Bloxham D, Saward R, Dremucheva A, Siebert R. Studies of four new human myeloma cell lines. Leuk Lymphoma. 2005;46:101–112. doi: 10.1080/10428190400002244. [DOI] [PubMed] [Google Scholar]
- 21.Kass GE, Orrenius S. Calcium signaling and cytotoxicity. Environ Health Perspect. 1999;107(Suppl 1):25–35. doi: 10.1289/ehp.99107s125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim R, Emi M, Tanabe K, Murakami S. Role of the unfolded protein response in cell death. Apoptosis. 2006;11:5–13. doi: 10.1007/s10495-005-3088-0. [DOI] [PubMed] [Google Scholar]
- 23.Kinnally KW, Antonsson B. A tale of two mitochondrial channels, MAC and PTP, in apoptosis. Apoptosis. 2007;12:857–868. doi: 10.1007/s10495-007-0722-z. [DOI] [PubMed] [Google Scholar]
- 24.Kramar R, Hohenegger M, Srour AN, Khanakah G. Oligomycin toxicity in intact rats. Agents Actions. 1984;15:660–663. doi: 10.1007/BF01966788. [DOI] [PubMed] [Google Scholar]
- 25.Kurtoglu M, Gao N, Shang J, Maher JC, Lehrman MA, Wangpaichitr M, Savaraj N, Lane AN, Lampidis TJ. Under normoxia, 2-deoxy-D-glucose elicits cell death in select tumor types not by inhibition of glycolysis but by interfering with N-linked glycosylation. Mol Cancer Ther. 2007;6:3049–3058. doi: 10.1158/1535-7163.MCT-07-0310. [DOI] [PubMed] [Google Scholar]
- 26.Landolfi B, Curci S, Debellis L, Pozzan T, Hofer AM. Ca2+ homeostasis in the agonist-sensitive internal store: functional interactions between mitochondria and the ER measured in situ in intact cells. J Cell Biol. 1998;142:1235–1243. doi: 10.1083/jcb.142.5.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin AM, Chao PL, Fang SF, Chi CW, Yang CH. Endoplasmic reticulum stress is involved in arsenite-induced oxidative injury in rat brain. Toxicol Appl Pharmacol. 2007;224:138–146. doi: 10.1016/j.taap.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 28.Malhotra JD, Kaufman RJ. The endoplasmic reticulum and the unfolded protein response. Semin Cell Dev Biol. 2007;18:716–731. doi: 10.1016/j.semcdb.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malli R, Frieden M, Osibow K, Zoratti C, Mayer M, Demaurex N, Graier WF. Sustained Ca2+ transfer across mitochondria is Essential for mitochondrial Ca2+ buffering, sore-operated Ca2+ entry, and Ca2+ store refilling. J Biol Chem. 2003;278:44769–44779. doi: 10.1074/jbc.M302511200. [DOI] [PubMed] [Google Scholar]
- 30.Malli R, Frieden M, Trenker M, Graier WF. The role of mitochondria for Ca2+ refilling of the endoplasmic reticulum. J Biol Chem. 2005;280:12114–12122. doi: 10.1074/jbc.M409353200. [DOI] [PubMed] [Google Scholar]
- 31.Masubuchi Y. Metabolic and non-metabolic factors determining troglitazone hepatotoxicity: a review. Drug Metab Pharmacokinet. 2006;21:347–356. doi: 10.2133/dmpk.21.347. [DOI] [PubMed] [Google Scholar]
- 32.Masubuchi Y, Kano S, Horie T. Mitochondrial permeability transition as a potential determinant of hepatotoxicity of antidiabetic thiazolidinediones. Toxicology. 2006;222:233–239. doi: 10.1016/j.tox.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 33.McPherson PS, Campbell KP. The ryanodine receptor/Ca2+ release channel. J Biol Chem. 1993;268:13765–13768. [PubMed] [Google Scholar]
- 34.Min SK, Lee SK, Park JS, Lee J, Paeng JY, Lee SI, Lee HJ, Kim Y, Pae HO, Kim EC. Endoplasmic reticulum stress is involved in hydrogen peroxide induced apoptosis in immortalized and malignant human oral keratinocytes. J Oral Pathol Med. 2008;37:490–498. doi: 10.1111/j.1600-0714.2008.00679.x. [DOI] [PubMed] [Google Scholar]
- 35.Nadanaciva S, Dykens JA, Bernal A, Capaldi RA, Will Y. Mitochondrial impairment by PPAR agonists and statins identified via immunocaptured OXPHOS complex activities and respiration. Toxicol Appl Pharmacol. 2007;223:277–287. doi: 10.1016/j.taap.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 36.Obeng EA, Carlson LM, Gutman DM, Harrington WJ, Jr, Lee KP, Boise LH. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood. 2006;107:4907–4916. doi: 10.1182/blood-2005-08-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oh-Hashi K, Maehara K, Isobe K. Hydrogen peroxide induces GADD153 in Jurkat cells through the protein kinase C-dependent pathway. Redox Rep. 2004;9:173–178. doi: 10.1179/135100004225005183. [DOI] [PubMed] [Google Scholar]
- 38.Ong HL, Liu X, Sharma A, Hegde RS, Ambudkar IS. Intracellular Ca(2+) release via the ER translocon activates store-operated calcium entry. Pflugers Arch. 2007;453:797–808. doi: 10.1007/s00424-006-0163-5. [DOI] [PubMed] [Google Scholar]
- 39.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 40.Reddy PS, Corley RB. The contribution of ER quality control to the biologic functions of secretory IgM. Immunol Today. 1999;20:582–588. doi: 10.1016/s0167-5699(99)01542-x. [DOI] [PubMed] [Google Scholar]
- 41.Rimessi A, Giorgi C, Pinton P, Rizzuto R. The versatility of mitochondrial calcium signals: from stimulation of cell metabolism to induction of cell death. Biochim Biophys Acta. 2008;1777:808–816. doi: 10.1016/j.bbabio.2008.05.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schewe DM, Aguirre-Ghiso JA. Inhibition of eIF2alpha dephosphorylation maximizes bortezomib efficiency and eliminates quiescent multiple myeloma cells surviving proteasome inhibitor therapy. Cancer Res. 2009;69:1545–1552. doi: 10.1158/0008-5472.CAN-08-3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 44.Tagawa Y, Hiramatsu N, Kasai A, Hayakawa K, Okamura M, Yao J, Kitamura M. Induction of apoptosis by cigarette smoke via ROS-dependent endoplasmic reticulum stress and CCAAT/enhancer-binding protein-homologous protein (CHOP) Free Radic Biol Med. 2008;45:50–59. doi: 10.1016/j.freeradbiomed.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 45.Van Coppenolle F, Vanden Abeele F, Slomianny C, Flourakis M, Hesketh J, Dewailly E, Prevarskaya N. Ribosome-translocon complex mediates calcium leakage from endoplasmic reticulum stores. J Cell Sci. 2004;117:4135–4142. doi: 10.1242/jcs.01274. [DOI] [PubMed] [Google Scholar]
- 46.Wang XZ, Lawson B, Brewer JW, Zinszner H, Sanjay A, Mi LJ, Boorstein R, Kreibich G, Hendershot LM, Ron D. Signals from the stressed endoplasmic reticulum induce C/EBP-homologous protein (CHOP/GADD153) Mol Cell Biol. 1996;16:4273–4280. doi: 10.1128/mcb.16.8.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Z, Yang XY, Cohen DM. Urea-associated oxidative stress and Gadd153/CHOP induction. Am J Physiol. 1999;276:F786–F793. doi: 10.1152/ajprenal.1999.276.5.F786. [DOI] [PubMed] [Google Scholar]