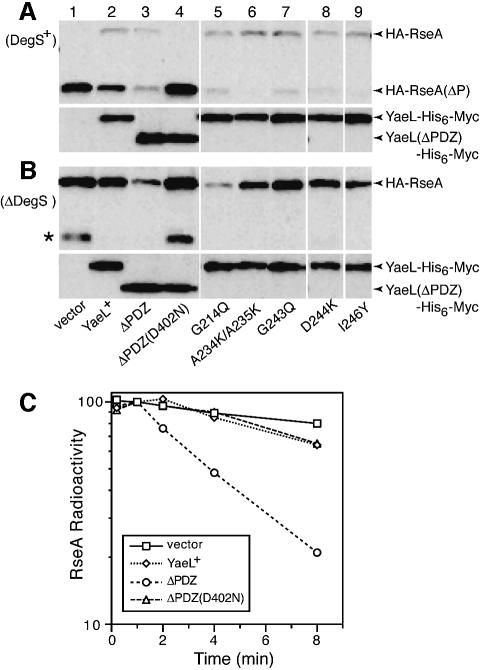
Fig. 2. Effects of YaeL PDZ mutations on degradation of RseAΔP and RseA. (A) RseA accumulation in the presence of DegS. Strain KK211 (ΔyaeL ΔrseA)/pSTD691 (HA-RseA) was further transformed with pTWV228 (vector, lane 1); pKK11 (YaeL+-His6-Myc, lane 2); pKK131 (YaeLΔPDZ-His6-Myc, lane 3); pKK135 [YaeLΔPDZ(D402N)-His6-Myc, lane 4], pKK138 [YaeL(G214Q)-His6-Myc, lane 5]; pKK139 [YaeL(A234K/A235K)-His6-Myc, lane 6]; pKK140 [YaeL(G243Q)-His6-Myc, lane 7]; pKK136 [YaeL(D244K)-His6-Myc, lane 8] and pKK141 [YaeL(I246Y)His6-Myc, lane 9]. Cells were precultured in L glucose (0.4%) medium, inoculated into M9 medium supplemented with 20 amino acids, 2 µg/ml thiamine, 0.4% glucose and 1 mM IPTG, and grown at 30°C for 3.5 h. Whole cellular proteins were subjected to SDS–PAGE and anti-HA (upper panels) and anti-Myc (lower panels) immunoblotting. (B) RseA accumulation in the absence of DegS. Strain AD1840 (ΔdegS ΔyaeL ΔrseA)/pSTD691 was further transformed with the same set of plasmids and processed as described in (A). Asterisk indicates a C-terminally cleaved product of HA-RseA that was produced by the action of some unknown periplasmic proteases and this truncated product is susceptible to active YaeL. (C) Stability of RseA in the absence of DegS. Strain AD1840/pSTD691 was further transformed with pTWV228, pKK11, pKK131 or pKK135. Cells were grown in M9 medium supplemented with 18 amino acids (other than Met and Cys), 2 µg/ml thiamine and 0.4% glucose at 30°C, and induced with 1 mM IPTG and 1 mM cAMP for 2 h. Cells were then pulse-labeled with [35S]methionine for 1.5 min followed by chase with unlabeled methionine for the indicated periods. Samples were processed for anti-HA immunoprecipitation. Radioactivities associated with HA-RseA were determined after SDS–PAGE and phosphorimaging, and are reported as values relative to the 1 min chase radioactivity for each culture set as 100%.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.