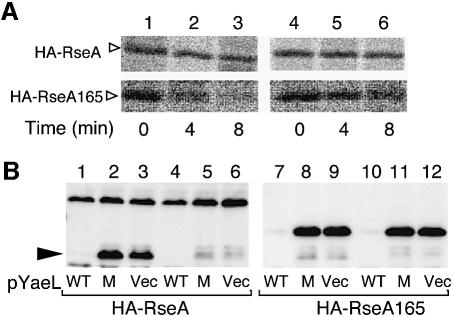
Fig. 7. YaeL-dependent and DegS-independent degradation of RseA165. (A) Cells of AD1839 (ΔdegS ΔrseA, lanes 1–3) and AD1840 (ΔdegS ΔyaeL ΔrseA, lanes 4–6), each carrying pKK55 (HA-RseA+, upper panels) or pSTD671 (HA-RseA165, lower panels) were grown in M9 medium supplemented with 18 amino acids (other than Met and Cys), 2 µg/ml thiamine and 0.4% glucose at 30°C, and induced with 1 mM IPTG and 1 mM cAMP. Cells were then pulse-labeled with [35S]methionine for 1 min followed by chase with unlabeled methionine for the indicated periods. Samples were processed for anti-HA immunoprecipitation, SDS–PAGE and phosphorimaging. (B) Strains KK211 (ΔyaeL ΔrseA)/pKK55 (HA-RseA) (lanes 1–3), AD1840 (ΔdegS ΔyaeL ΔrseA)/pKK55 (lanes 4–6), KK211/pSTD671 (HA-RseA165) (lanes 7–9) and AD1840/pSTD671 (lanes 10–12) were transformed further with one of the following plasmids: pSTD630 (YaeL+-His6-Myc; WT) for lanes 1, 4, 7 and 10; pSTD631 [YaeL(H22F)-His6-Myc; M] for lanes 2, 5, 8 and 11 and pMPM-T3 (vector; V) for lanes 3, 6, 9 and 12. Plasmid-bearing cells were grown at 30°C in L-broth containing 1 mM IPTG and 1 mM cAMP. Total cellular proteins were subjected to SDS–PAGE and anti-HA immunoblotting.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.