Abstract
Aims and Objectives
Many natural health products (NHPs) and dietary supplements (DS) are purchased in pharmacies and it has been argued that pharmacists are in the best position to provide patients with evidence-based information about them. This study was designed to identify how the pharmacist’s role with respect to NHPs/DS is portrayed in the literature.
Method
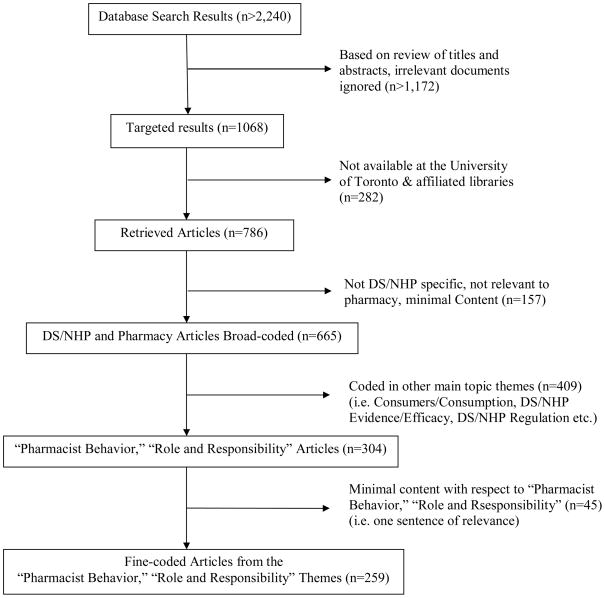
A systematic search was conducted in a variety of health databases to identify all literature that pertained to both pharmacy and NHPs/DS. Of the 786 articles identified, 665 were broad-coded and 259 were subjected to in-depth qualitative content analysis for emergent themes.
Key Findings
Overwhelmingly, support for the sale of NHPs/DS in pharmacies is strong. Additionally, a role for pharmacists in NHP/DS counselling is underscored. But another recurrent theme is that pharmacists are ill-equipped to counsel patients about these products that are available on their shelves. This situation has led some to question the ethics of pharmacists selling NHPs/DS and to highlight the existence of an ethical conflict stemming from the profit-motive associated with NHP/DS sales.
Conclusion
This analysis raises concerns about the ethics of NHPs/DS being sold in pharmacies, and about pharmacists being expected to counsel about products of which they have little knowledge.
1.0 Introduction
Pharmacists are an integral part of the conventional health care team within the context of the North American health care system. There is some variability of regulations governing pharmacists among Canadian provinces and American states; however, all are bound by national drug regulations. The role of pharmacists generally includes “the custody, compounding and dispensing of drugs, the provision of non-prescription drugs, health care aids and devices and the provision of information related to drug use.” (1) The introduction of new categories of products – natural health products (NHPs) in Canada and dietary supplements (DS) in the United States and has created new challenges for pharmacists striving to provide comprehensive patient-centred pharmaceutical care for patients.
In 1994, the Dietary Supplement Health and Education Act (DSHEA) came into effect in the U.S.A (2). In it, a DS is defined as “a product (other than tobacco) that is intended to supplement the diet that bears or contains one or more of the following dietary ingredients: a vitamin, a mineral, an herb or other botanical, an amino acid, a dietary substance for use by man to supplement the diet by increasing the total daily intake, or a concentrate, metabolite, constituent, extract, or combinations of these ingredients”. More recently, in 2007 the U.S. Food and Drug Administration introduced good manufacturing practices (cGMP) for DS (3). This regulation “ensures that dietary supplements are produced in a quality manner, do not contain contaminants or impurities, and are accurately labelled”, thus strengthening the pre-existing regulations.
Similarly, a new category called NHPs has been recognized in Canadian legislation since January 1, 2004.(4) The definition of NHPs is: natural source “substances which are manufactured, sold or represented for use in: i) the diagnosis, treatment, mitigation or prevention of a disease, disorder, or abnormal physical state or its symptoms in humans; ii) restoring or correcting organic functions in humans; or iii) maintaining or promoting health or otherwise modifying organic function in humans” (p. 1536). Medicinal ingredients to be regulated under the new regulations are listed in an inclusion list (e.g., plant or plant material, algae, fungus or non-human material, vitamins, amino acids, essential fatty acids, minerals, homeopathic preparations and traditional medicines). All products covered by the new regulations must be in dosage forms (i.e., bulk herbs are not included) and must have a wide margin of safety. By definition, NHPs are safe for over-the-counter sale directly to consumers.(5)
Although NHPs and DS are widely available in pharmacies across North America, there has been relatively little discussion among members of the profession about what professional responsibilities pharmacists have with respect to these products. Pharmacists’ responsibility to detect and prevent interactions between NHPs/DS and conventional medications has consistently been identified in the literature as important,(6–9) and a recent North American study suggested that use of prescription drugs in conjunction with NHPs/DS is high enough (16%) to raise concerns about unintended interactions.(10)
Several information papers suggest that pharmacists should provide objective information to help patients make informed choices about DS/NHP use.(11, 12) These reports seem to imply that there is relative agreement within the profession of pharmacy, not only that pharmacists have professional responsibilities with respect to NHPs/DS, but also with respect to key aspects of what those responsibilities are (or should be). However, there is evidence that this is not an accurate portrayal of the opinions and practices within the profession. For example, a study of US pharmacists reported that although almost three-quarters of the pharmacists surveyed worked in a retail setting where herbal medicines were sold, almost half agreed with the statement, “herbal medicines are not accepted by the majority of my colleagues” and only a quarter agreed with the statement “herbs are efficacious.”(13) A recent Canadian study reported that only 2% of pharmacists felt they had adequate information about complementary and alternative health care.(14) Furthermore, we know from a systematic review of surveys of pharmacists that opinions within the profession are mixed, with roughly half reporting positive attitudes toward NHPs/DS, and the other half reporting negative attitudes.(13) Pharmacists’ practices also lag behind at least some of expectations outlined in the information papers, since most pharmacists do not routinely document, monitor, or inquire about patients’ use of NHPs or DS.(13)
The purpose of this study was to complete a comprehensive, systematic assessment of the pharmacy literature to identify how the pharmacist’s role with respect to NHPs and DS is portrayed. This work forms the first step in a larger study which aims to develop core competencies for undergraduate pharmacy students with respect to NHPs and DS that can be implemented at pharmacy schools across North America.
2.0 Methods
A systematic, comprehensive literature search and qualitative analysis were completed for this study. Each is detailed below.
2.1 Literature Search
In effort to gain a comprehensive picture of literature pertaining to NHPs/DS and pharmacists, three search strategies were employed using the criteria outlined in Table 1. First, a search was performed in a number of medical and pharmaceutical databases from inception to February 2006. Databases included: Medline (Ovid 1966 – February 2006), CiNahl (Ovid 1982 – 2006), International Pharmaceutical Abstracts (Ovid 1970 – 2006), Sociological Abstracts (Scholars Portal 1960–2006). For the purposes of this paper, articles were not sought from Health Star (Ovid 1966 – 2006) and Embase (Ovid 1980 – 2006) for two reasons: (1) in order to make the fine-coding manageable, we chose only to utilize a smaller subset of available articles; (2) the databases chosen for inclusion represent the bulk of literature published in a North American context and were consequently deemed the most important for adequate coverage; consequently, Embase and Healthstar were deemed of secondary importance.
Table 1.
Pharmacy & DS/NHP Documentary Collection Strategy
Second, a keyword search was performed directly in the Canadian Pharmacists/Pharmaceutical Journal (CPJ/RPC) website. Results yielded from these searches were assessed for topics regarding pharmacists and NHPs/DS (i.e. articles containing the words “pharmacist” or “pharmacy” in addition to words pertaining to NHPs/DS or articles in a pharmaceutical journal pertaining to NHPs/DS). Articles deemed to be of relevance, based on these criteria, were obtained by downloading electronically or photocopying by hand. Third, in a final attempt to acquire relevant information, when collecting articles (hardcopy or electronically), neighbouring articles of relevance within the same journal (that were not picked up in the search strategies used) were also obtained for review.
Due to the variety of terms that relate to NHPs/DS, a number of keywords were employed in the searches. Search terms were related to natural health products, botanicals, vitamins, minerals, supplements, homeopathic, complementary or alternative medicine and pharmacists and pharmacies. Refer to Table 2 for an overview of search terms and search strategies used.
Table 2.
Database Search Strategies
| Database/Journal | Subject Headings3 | Key Word Searches4 |
|---|---|---|
| Canadian Pharmacists/Pharmaceutical Journal (CPJ/RPC) (all years) | [not available] | [entered individually] ‘alternative medicine’, ‘complementary medicine’, ‘herbal medicine’, ‘dietary supplements’, ‘vitamins’ ‘minerals’, ‘homeopathy’, ‘homeopathic’ |
| CiNahl (Ovid 1982 – February 2006) | ‘natural product’, ‘alternative therapies’, ‘pharmacists’, ‘pharmacy and pharmacology’ | (herb$ or (natural health product$) or mineral$ or vitamin$ or supplement$ or probiotic$ or homeopath$ or botanic$ or (complementary medicine$) or (alternative medicine$) or (complementary health$) or (alternative health$) or (complementary therap$) or (alternative therap$)).hw AND (pharmacy or pharmacist$ or pharmacies or pharmaceutical$).hw |
| International Pharmaceutical Abstracts (Ovid 1970 – February 2006) | [not available] | [same as CiNahl] |
| Medline (Ovid 1966 – February 2006) | ‘Plant Preparations’, ‘Plant Extracts’, ‘Dietary Supplements’, ‘Phytotherapy’, ‘complementary therapies’, ‘pharmacists’, ‘pharmacy’ | [same as CiNahl] |
| Sociological Abstracts (Scholars Portal 1960 – February 2006) | ‘alternative medicine’ [no search heading for ‘pharmacy/pharmacist’] | (KW=pharma*) and ((KW=(herb* or (natural health product) or mineral*) or KW=(vitamin* or supplement* or probiotic*) or KW=(homeopath* or botanic*)) or (KW=((complementary medicine) or (alternative medicine) or (complementary health*)) and KW=((alternative health*) or (complementary therap*) or (alternative therap*)))) |
2.2 Qualitative content analysis
The documents were analyzed using qualitative content analysis.(15, 16) The analysis consisted of two steps: 1) coding of the topic of article content and 2) detailed coding of the articles in selected content areas that contained adequate content (i.e. more than 1 line of relevant text). In step 1, three investigators (HB, KH, and MC) met together to review a random sample of the articles to identify “main topic” themes. We continued coding articles together until we were confident that we had created a coding tree which could be used to identify the main topics of all the articles identified in the literature search. One investigator (MC or DG) was then able to code the main topics of the remainder of the articles.
In step 2, fine coding of a sub-set of articles with main and sub-topics coded within the themes “Pharmacist role and responsibility” and “Pharmacist Behaviour” (i.e., those that contained more than a single statement describing or implying a role for the pharmacist with respect to NHPs/DS) were coded in more detail.
To begin, nine articles representing a range of the types of articles in the two main topics were selected and their content was assessed independently by the three investigators (HB, KH, MC). A second meeting was held to combine independent assessments and determine a detailed coding scheme. Using this coding scheme, a new sample of four articles was coded independently by two investigators (KH and MC). This was followed by a final team meeting to address any inconsistencies in coding and to fine-tune the detailed coding scheme. The remaining articles were coded by one investigator (MC) who consulted with the others when necessary during the coding process. Themes were coded using QSR NVIVO 7 to organize the data and support qualitative analysis.
3.0 Results
As indicated in Table 1, our search found 2240 articles. A total of 665 articles were broad coded into region of origin, document type and topic (including main and sub-topic). Of the 304 articles that fell into categories relevant to assessing pharmacists’ potential role(s), 259 articles with adequate content were in turn selected for fine-coding (references available upon request from the authors).
3.1 Broad Coding of Documents
At the first stage of analysis, broad document coding was undertaken with the 665 articles included in this study. Specifically, codes that represented the following themes were applied to each article: ‘document type’, ‘document topic’ and ‘country of origin’. The vast majority of documents were feature/report articles (n=321; 48%) which include news/magazine articles, special reports in journals, interviews, opinion/point of view statements, and journal supplements. The second most frequent type of documents we identified were review articles (n=185; 28%), which were divided into the sub-categories of DS/NHP profiles (n=113; 17%) and general review (n=72; 11%). The former category includes articles that reviewed literature pertaining to specific NHPs/DS, typically covering the use, safety and efficacy or effectiveness of these NHPs/DS. The latter category includes systematic and narrative review articles of a more general nature that summarize the current state of research and knowledge about multiple NHPs/DS, including consumption patterns and trends. Primary research (n=81; 12%) is the third most frequent type of document we analyzed. It includes both social science research (n=78; <12%) and a small number of clinical/basic research (n=3; <1%) articles. These categories are followed by letters (n=26; 4%), continuing education excerpts (n=19; 3%), editorials (n=15; 2%), commentaries (n=11; 2%), and finally, conference announcements and proceedings (n=7; 1%).
We provide tallies for the main topic of the articles here. By far the most frequent main topic was DS/NHP-specific articles (n=398; 60%) that focused either on single types of NHPs/DS (e.g., herbals or vitamins and minerals), or in a much smaller number of cases, multiple types of NHPs/DS1. Not surprisingly, safety (n=96; 14%) was the next most frequent main topic, followed by pharmacist behaviour (n=75; 11%), which includes references to what pharmacists are currently doing with respect to NHPs/DS. Next in frequency is the topic of regulation (n=74; 11%), disease/condition specific profiles (n=72; 11%) that review NHPs/DS as treatment options, as well as a focus on pharmacists’ current or potential roles/responsibilities (n=71; 11%). These are followed by evidence/efficacy (n=65; 10%), consumption (n=55; 8%), pharmacy education (n=37; 6%), general CAM (complementary and alternative medicine) articles (n=36; 5%), a population specific focus (31; 5%), marketing (n=23; 3%), quality (n=22; 3%), profits (n=18; 3%), and finally, existing models/protocols of integration (n=13; 2%).
Of the subset of 304 documents whose main topic or sub-topic are pharmacist behaviour or pharmacist roles/responsibilities, the vast majority are feature/report articles (164), followed by social science research (60) and general reviews (33 respectively)2. The remainder of articles in these topics were scattered among the other document types.
Finally, of those 665 documents reviewed, 279 (42%) originated from the U.S., 244 (37%) originated from Canada, 71 (11%) from the UK and 37 (6%) from Australia. This distribution was expected, given our focus on English language literature.
3.2 Fine Coding of Documents According to Emergent Themes
Here we review the themes of whether “pharmacists have a role with respect to NHPs/DS” and whether “pharmacists should be selling NHPs/DS”. Following these themes, we explore the “ethics of the message” that emerges in this literature.
3.2.1 Do Pharmacists Have a Role with Respect to NHPs/DS?
Most of the literature either explicitly or implicitly identified that pharmacists have a key role to play with respect to NHPs/DS. Support for counselling about NHPs/DS as a key role for pharmacists was extremely wide-spread and general. Indeed, the vast majority of content reviewed contained reference to pharmacists’ counselling role, implying that most authors assumed pharmacists should be involved in this area:
Although pharmacists may be reluctant to discuss the use of herbal therapies with patients for fear that such discussion may imply endorsement or approval, it is nonetheless the pharmacist’s professional responsibility to advise patients about potentially harmful aspects of herbal remedies, including possible interactions or contraindications with synthetic medications and/or current disease states. To do otherwise could leave patients at risk for major problems and jeopardize the public trust accorded pharmacists. (17)
A very common sub-theme was that pharmacists’ role(s) arose from consumer demand for NHPs/DS. The general argument was that since so many patients are using these products, pharmacists need to have some knowledge in this area:
However, it seems inevitable that patients’ increasing demand for and use of alternative therapies will continue to increase the need for information and advice from pharmacists, particularly regarding alternative therapies that are medicinal in nature. In fact, the current findings, along with other research, indicate that pharmacists are already experiencing an increased demand for such information, especially in pharmacies that stock herbal medicines. (18)
Another variation on this theme was that pharmacists are one of the most accessible health care practitioners and they are available at the point of sale:
The availability of these products in many pharmacy settings and the rapidly growing body of knowledge regarding the potential for significant interactions between drugs and natural products have led to the emergence of pharmacists as a “front line” for provision of information regarding the safety and efficacy of natural products. (19)
Several authors made the point that NHPs/DS were the future of pharmacy:
In the future pharmacists will need to be the information providers for more than just conventional pharmaceuticals and medical devices. Those pharmacists that do not continue to move forward and keep up, particularly in herbal medicine and especially in combination with conventional medications, could put their patients in danger and the reputation of their profession at risk. (20)
However, not everyone agreed that NHPs/DS should be part of pharmacists’ scope of practise:
As the columns of The Pharmaceutical Journal have shown in recent years, some pharmacists would ban from pharmacies all herbal and homoeopathic products that have not been validated by clinical trials. (21)
…skeptics declare that pharmacists have no business selling and promoting products whose medical worth is still in question. (22)
Others simply questioned what role pharmacists can have when there is evidence that patients are not asking pharmacists for advice:
However, even if pharmacists were standing ready to advise patients in their use of herbals or to advise not to use - there is no guarantee that patrons will request information or assistance from a pharmacist in selecting an herbal product. One study showed that 72% of respondents who used alternative medicine (including herbals) did not inform any health care professional of their use [in reference to Eisenberg et al., 1993]. (13)
Overwhelmingly, however, the message that emerges in these documents is that pharmacists do, indeed, have a role to play with respect to NHPs/DS, particularly with respect to counseling.
3.2.2 Should pharmacists sell NHPs?
Most documents made the point that the DS/NHP (and especially the herbal) market is growing overall and within pharmacies. There was almost unanimous support that NHPs/DS should be sold in pharmacies (the only disagreement was over the sale of homeopathy). Some of the documents were clearly intended to encourage pharmacists to sell NHPs, extolling the good profit margins of these products
A large proportion of documents on this topic made the point that NHPs/DS are profitable for pharmacies, highlighting the age-old challenge that pharmacists face because they are both health care professionals and also business people expected to generate a profit:
Good health care outcomes mean satisfied customers/patients who gladly keep their business where their good health lies. This leads to increased sales, in both natural medicine as well as prescriptions. (23)
The outlook for this market niche is optimistic and by incorporating alternative medicine in these services, pharmacists can be assured that their profit margin will increase. (24)
The profit motivation for selling NHPs/DS was further highlighted in articles that described niche pharmacists/pharmacies that specialized in these products. This is not to suggest that profit was the only motivation for DS/NHP sales in pharmacies. The following author, for example, highlights that patient-centered care is the justification for carry these products:
[F]inancial gain is not the main reason pharmacists get involved. As in any profession, there are, no doubt, some pharmacists who see the alternative medicine movement as a “cash cow.” Most pharmacists, however, focus more on their patients and what they are using, particularly if it has the potential for disrupting the benefits of their medication regimens. Thinking that any pharmacist who has an interest in learning more about alternative therapies is doing so just to make money is an unfair and, most likely, erroneous assumption. (25)
It was also clear from the documents that DS/NHP sales are already a core part of pharmacy business and that this decision may be made by corporate headquarters rather than by an individual pharmacist working at store level:
In the real world, many pharmacists don’t have the option of remaining aloof from the issue. If they are employees, they will have to deal with the reality that herbal products will very likely be sold in their store. And if they own their own pharmacy, they have to decide whether they should refuse to sell herbals on principle--and thereby lose profits while leaving their patients to the mercies of possibly unskilled health food store staff. (22)
Many pharmacists do not consequently make decisions about the products carried in their workplaces, and are simply left to make the best of this situation that we would characterize as an “ethical conflict” as described below.
3.2.3 The Ethics of the Message: An Emerging Ethical Conflict?
Although there is widespread support that pharmacists should be selling NHPs/DS and that patient counselling about these products is an important and growing part of the pharmacists’ role, some authors pointed to the emergence of ethical issues. There were two main ethical conflicts identified in the documents. The first is the issues raised by the sale of products for which there is not scientific evidence of effectiveness or safety. This issue is generally described in terms of consumers’ enhanced choice being in conflict with the pharmacists’ duty to help and protect the patient. The following quotations sum up this first ethical issue:
Is it unethical for pharmacies to carry and promote herbal remedies? Is it unethical for traditional pharmaceutical companies to lend their trusted names to these dietary products? Is it unethical for pharmaceutical laboratories to support these products by certifying standardization of constituents while knowing that efficacy studies are lacking? These are just a few questions that pharmacy must address in the years to come. The immediate dilemma that today’s pharmacist faces with alternative medicine lies in determining what really works and what does not. And how does one determine the truth about the medical safety and efficacy of any herbal product in today’s market? (26)
A pharmacist may not recommend a product whose safety or quality is in doubt, but there is nothing, apparently, to prevent him or her selling dubious remedies. (21)
Homeopathy was a special case of this first ethical issue that was highlighted in several articles because of the theoretical incompatibility of homeopathic principles with conventional pharmacology:
[T]he incompatibility of homeopathic paradigms with all of basic science must be appreciated. Although some pharmacists may wish to keep an “open mind” about the use of such dubious products, they should be reminded that the 1996 revision of The Ontario College of Pharmacists Code of Ethics clearly states that, “pharmacists never knowingly condone the dispensing, promoting or distributing of drugs… which are not of good quality”.(f.23) (27)
The second issue identified here is whether it is ethical for pharmacists to sell NHPs/DS if they do not have the knowledge to counsel patients about them (including knowledge about evidence that is in existence):
Pharmacists have an ethical responsibility for currency of knowledge about any medicines that they offer for sale or supply to the public. (28)
Even where ethics was not identified explicitly with respect to lack of knowledge or evidence, there are numerous passages that highlight this gap in pharmacy practice and education:
Pharmacists know that our patients are using these products, yet we have little understanding of them. Lack of knowledge about interactions and dosage and administration is a major concern and will continue to be if we do not develop a scientific body of knowledge to address these issues. (29)
Health care providers should be concerned about the lack of scientific evidence present to make a solid decision about supplements. (30)
What becomes apparent in the passages quoted here is that an information or knowledge gap is either the source of an ethical conflict, or compounds an already existing ethical conflict between patient care and profit. Whereas a lack of information focuses on the lack of research and evidence, the knowledge gap refers to pharmacists’ lack of knowledge even about what information and evidence does currently exist. Despite the identification of these information/knowledge gaps and the raising of related ethical concerns by some, the overwhelming message in these documents has still been that pharmacists should have a role and should be selling NHPs/DS.
4.0 Discussion and conclusions
4.1 Summary of Findings
There are three main arguments provided in the literature that support pharmacists’ having a role with respect to NHPs/DS: safety concerns, consumer demand for these products, and the current availability of these products in pharmacies. Two main arguments are given for why pharmacists should not (necessarily) undertake a role: the lack of evidence of efficacy of many of these products and the fact that many patients are not asking (pharmacists) for advice. These concerns notwithstanding, the message is clear that NHPs/DS belong in pharmacy practice. There is also overwhelming support in the literature for pharmacists selling NHPs/DS, despite concerns raised about the ethics of selling and promoting products for which there is limited evidence of efficacy and about which pharmacists know very little.
In particular, we highlighted two ethical conflicts: the first relates to the conflict between enhancing patients’ choice around access to NHPs/DS and the pharmacists’ duty to help and protect the patient, including ensuring that they only sell products proven to be efficacious and safe. The context for this is the growing market for DS/NHP sales and the profit motive associated with carrying these products in pharmacies. A related concern is the symbolic message that the sale of these products in pharmacies sends to the public, i.e. that these products are credible by virtue of being in a pharmacy. The second ethical conflict pertains to pharmacists being in a position to sell and promote these products despite an apparent overall lack of knowledge about them, which amplifies the already existing ethical dilemma associated with pharmacy being a merchandizing profession, i.e. being involved in product sales. What complicates these issues immensely is that merchandizing decisions are often not made by pharmacists working directly in the pharmacies with these products. The result is that most community pharmacists are simply left to navigate ethical conflicts that they themselves are not responsible for creating.
4.2 Study Strengths and Limitations
This is the largest and most rigorous analysis of the pharmacy literature with respect to to pharmacists’ roles with respect to NHPs and DS. However, the state of science in this area is changing rapidly, and the literature reviewed in this study largely pre-dates the phasing in of NHP regulations beginning in 2004 in the Canadian context and changes such as the introduction of good manufacturing standards for DS in the USA. This means that safety and evidence status for many of these products may have changed in the interim. A further limitation of the analysis conducted here is its focus on English-only articles, and additionally, its limitation to databases of most relevance to North American literature. As noted previously, the decision not to undertake content analysis of articles in Health Star and Embase was made both to make the task more manageable as well as to highlight the North American context of pharmacy practice to inform our ongoing larger project in this area. The obvious result is that trends in other national contexts may have been missed.
4.3 International Relevance
While this systematic documentary analysis included any literature published in English, practical decisions about the search strategy resulted in a focus on North American pharmacy literature. However, there is ample evidence that the findings from this study may be relevant to pharmacists in industrialized countries around the world. There are articles discussing pharmacists’ roles with respect to NHPs/DS in many countries including Finland(31), Japan(32), Spain(33), Australia(34, 35), Thailand(34), and the United Kingdom.(36, 37) Most explicitly or implicitly endorse the idea that NHPs/DS should be sold in pharmacies;(31–34, 38) although as we found in our review, there are differing opinions about whether pharmacists should recommend these products.(31, 32) Issues related to increasing use of these products and pharmacists’ lack of knowledge about these products appear to be facing pharmacists everywhere.(32, 34)
4.4 Conclusion
In conclusion, the notable message that emerges in these documents – with a small number of detractors – is that pharmacists do have a role to play with respect to NHPs/DS and that they should be selling NHPs/DS. What is problematic about this message – beyond the concerns raised about the information and knowledge gap confounding existing issues – is that it is largely deterministic and circular: patient demand for NHPs/DS exists, therefore pharmacies should be selling NHPs/DS, and since pharmacies are already selling NHPs/DS, pharmacists should have a role with respect to NHPs/DS (e.g. they should counsel about these products).. However, none of these conclusions necessarily or logically follow from the ones before, leading us to argue that open debate and discussion about the merits of pharmacists undertaking a role has largely been precluded by this line of thinking. While the message in these documents might be clear-cut (if presumptuous), it by no means represents the profession of pharmacy at large or even the views of most practicing pharmacists. It is simply what we called it – ‘a message’ – granted a powerful and one-sided message that charts a particular future course for pharmacy, but a future course that is not wholly determined. This message strongly underscores the need to consult with practicing pharmacists, pharmacy educators and policy-makers in order to foster debate about the issues raised here and ensure that pro-active decision-making, rather than abandonment to the determinism suggested by the status quo, characterize the future of pharmacy practice.
Acknowledgments
We would like to acknowledge our co-investigators that were part of the grant application of which this project was a part: Peri Ballantyne, Trent University; Timothy Caulfield, Faculty of Law, University of Alberta (U of A); Lynda Eccott, Faculty of Pharmaceutical Sciences, University of British Columbia; Marilyn Wang, Program Policy Branch, Ontario Ministry of Health and Longterm Care; Shirley Heschuk, Faculty of Pharmacy and Pharmaceutical Sciences, U of A; Tannis Jurgens, College of Pharmacy, Dalhousie University; Ken Potvin, Executive Director, National Association of Pharmacy Regulatory Authorities; Michael Smith, Natural Health Products Directorate; Sandy Welsh, Department of Sociology, University of Toronto; Deanna Williams, Registrar, Ontario College of Pharmacists. Heather Boon was supported by a Canadian Institute of Health Research (CIHR) new Investigator award and Kristine Hirschkorn was supported by a CIHR Doctoral Fellowship during the completion of this research. This project was funded by a CIHR Partnerships for Health System Improvement grant.
Footnotes
Note that articles may have been coded into more than one ‘topic’ category.
Note again that there is overlap between the topics.
‘Subject Headings’ refer to those search headings that are provided a priori by the respective databases, based on their strategies for categorizing materials
‘Key Word Searches’ refer to the individual search terms [and strings of search terms] entered into the databases by the researchers
5.0 References
- 1.Pharmacy Act (Province of Ontario). 1991.
- 2.Dietary Supplement Health and Education Act of 1994. Nutrition USFaDA-CfFSaA. 1994.
- 3.FDA Issues Dietary Supplements Final Rule. Administration USFaD. 2007.
- 4.Her Majesty the Queen in Right of Canada. Natural Health Products Regulations. Canada Gazette Part II. 2003 Jun 18;137(13) [Google Scholar]
- 5.Federal Government of Canada. Natural Health Product Regulations. Ottawa: Department of Health; 2003. [Google Scholar]
- 6.Jurgens T. Who should be providing information to patients about herbal medicine? Canadian Journal of Clinical Pharmacology. 2001;8(4):186–7. [PubMed] [Google Scholar]
- 7.Levy S. What they’re asking about herbs - And what you can tell them. Drug Topics. 2000;144(2):42–4. [Google Scholar]
- 8.Cardinale V. How you and your patients can stay out of botanical trouble. Drug Topics. 2000;144(3):70–2. [Google Scholar]
- 9.Chavis LM. Pharmacy-based consulting on dietary supplements. Journal of the American Pharmaceutical Association. 2001;41(2):181–91. doi: 10.1016/s1086-5802(16)31239-6. [DOI] [PubMed] [Google Scholar]
- 10.Kaufman Recent patterns of medication use in the ambulatory adult population of the United States. The Slone Survey. Journal of the American Medical Association. 2002;287(3):337–44. doi: 10.1001/jama.287.3.337. [DOI] [PubMed] [Google Scholar]
- 11.Boon H for the CSHP Alternative Medicine Task Force; Canadian Society of Hospital Pharmacists. CSHP Official Publications 2001. Ottawa: Canadian Society of Hospital Pharmacists (CSHP); 2001. Information Paper: The role of the pharmacist with respect to complementary/alternative medicine; pp. 181–6. [Google Scholar]
- 12.Inter-Provincial Pharmacy Regulatory Committees of NAPRA. NAPRA Position Statements. Pharmacist’s Responsibility in Providing Advice About or Selling Alternative Health Products. [Internet website: http://www.napra.org/practic/information/response.html] 1999 [cited 2002 February 21]; Available from.
- 13.Bouldin AS, Smith MC, Garner DD, Szeinbach SL, Frate DA, Croom EM. Pharmacy and herbal medicine in the US. Social Science and Medicine. 1999;49:279–89. doi: 10.1016/s0277-9536(99)00118-5. [DOI] [PubMed] [Google Scholar]
- 14.Montbriand MJ. Alternative therapies. Health professionals’ attitudes. Canadian Nurse. 2000;96(3):22–6. [PubMed] [Google Scholar]
- 15.Boyatzis R. Transforming Qualitative Information: Thematic Analysis and Code Development. Thousand Oaks, CA: Sage; 1998. [Google Scholar]
- 16.Morse J, Field P. Qualitative Research Methods for Health Professinals. 2. Thousand Oaks, CA: Sage Publications; 1995. [Google Scholar]
- 17.Bennett J, Brown CM. Use of herbal remedies by patients in a health maintenance organization. Journal of the American Pharmaceutical Association. 2000 May-Jun;40:353–8. doi: 10.1016/s1086-5802(16)31082-8. [DOI] [PubMed] [Google Scholar]
- 18.Brown CM. Use of alternative therapies and their impact on compliance: perceptions of community. Journal of the American Pharmaceutical Association. 1998;38(5):603–8. doi: 10.1016/s1086-5802(16)30374-6. [DOI] [PubMed] [Google Scholar]
- 19.Clauson K, McQueen C, Shields K, Bryant P. Knowledge and attitudes of pharmacists in Missouri regarding natural products. American Journal of Pharmaceutical Education. 2003;67(2):1–9. [Google Scholar]
- 20.Rowell DM, Kroll DJ. Complementary and alternative medicine education in United States pharmacy schools. American Journal of Pharmaceutical Education. 1998;62(4):412–9. [Google Scholar]
- 21.Sturgess R. Is it Possible to Move Towards a Consensus on Homeopathy? The Pharamceutical Journal. 2002 Jul 27;269:138. [Google Scholar]
- 22.Johne A, Brockmoller J, Bauer S, Maurer A, Langheinrich M, Roots I. Pharmacokinetic interaction of digoxin with an herbal extract from St John’s wort (Hypericum perforatum) Clinical Pharmacology & Therapeutics. 1999;66(4):338–45. doi: 10.1053/cp.1999.v66.a101944. [DOI] [PubMed] [Google Scholar]
- 23.Grauds C. Clinical pharmacist talks to plants, too. Alternative Therapies in Health & Medicine. 2001;7(4):19–20. [PubMed] [Google Scholar]
- 24.Kattis T. Alternative medicine and its impact on the pharmacy profession. CPJ/RPC. 1999 Apr;132(3):11, 7. [Google Scholar]
- 25.Montagne M. Myths & realities of alternative therapies. US Pharmacist. 1999 Dec;24:56, 63–4, 7. [Google Scholar]
- 26.Williamson JS, Wyandt CM. The herbal generation: Trends, products, and pharmacy’s role. Drug Topics. 1999;143(7):69–78. [Google Scholar]
- 27.Harrison A, Pablo A, Verhoef M. The consumer’s role in co-ordination: Making sense of transitions in health care. In: Mark A, Dopson S, editors. Organizational Behaviour in Health Care - the Research Agenda. London, England: MacMillan Press Ltd; 1999. pp. 47–62. [Google Scholar]
- 28.Blenkinsopp A. St John’s wort - An ethical dilemma? Pharmaceutical Journal. 2005;274(7340):296. [Google Scholar]
- 29.Beal FC. Herbals and homeopathic remedies as formulary items? American Journal of Health-System Pharmacy. 1998;55:1266–7. doi: 10.1093/ajhp/55.12.1266. [DOI] [PubMed] [Google Scholar]
- 30.Semaan N. Integration of complementary disciplines into the oncology clinic. Part III. Herbal medicine. Current Problems in Cancer. 2000;24(4):213–22. doi: 10.1016/s0147-0272(00)90020-5. [DOI] [PubMed] [Google Scholar]
- 31.Fock J, Pietila K. The attitudes and opinions of pharmacy owners toward naural health products in Finland. Journal of Social and Administrative Pharmacy. 2003;20(6):242–8. [Google Scholar]
- 32.Shimizu R, Sakamoto Y, Nishizawa T, Iguchi S, Yamaoka Y. Survey of current conditions regarding awareness of the nutirtional role of supplements for pharmacy students. Journal of the Pharmaceutical Society of Japan. 2007;127(9):1461–71. doi: 10.1248/yakushi.127.1461. [DOI] [PubMed] [Google Scholar]
- 33.La-Cave A, Santana S, Sevillano L, Rodriguez N, Montelongo M, Garcia T, et al. Estimation of vitamin supplements and minerals consumption dispensed through pharmacy offices in the province of Las Palmas. Anales de medicina Interna. 2005;22(10):469–72. doi: 10.4321/s0212-71992005001000004. [DOI] [PubMed] [Google Scholar]
- 34.Kanjanarach T, Krass I, Cumming R. Exploratory study of factors influencing practice of pharmacists in Australia and Thailand with respect to dietary supplements and complementary medicine. International Journal of Pharmacy Practice. 2006;14(2):123–8. [Google Scholar]
- 35.Brownie S, Rolfe M. Supplement utilization patterns of older Australians: results from a randomly selected national sample. Nutrition and Dietetics: Journal of the Dietitians Association of Australia. 2005;62(2/3):89–94. [Google Scholar]
- 36.Barnes J. Pharmacists must not shun complementary health approaches. The Pharmaceutical Journal. 2002;268:359. [Google Scholar]
- 37.Barnes J. Pharmacovigilance of herbal medicines: a UK perspective. Drug Safety. 2003;26(12):829–51. doi: 10.2165/00002018-200326120-00001. [DOI] [PubMed] [Google Scholar]
- 38.Barnes J, Mills S, Abbot N, Willoughby M, Ernst E. Different standards for reporting ADRs to herbal remedies and conventional OTC medicines: face-to-face interviews with 515 users of herbal remedies. British Journal of Clinical Pharmacology. 1998;45:496–500. doi: 10.1046/j.1365-2125.1998.00715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]