Figure 5.
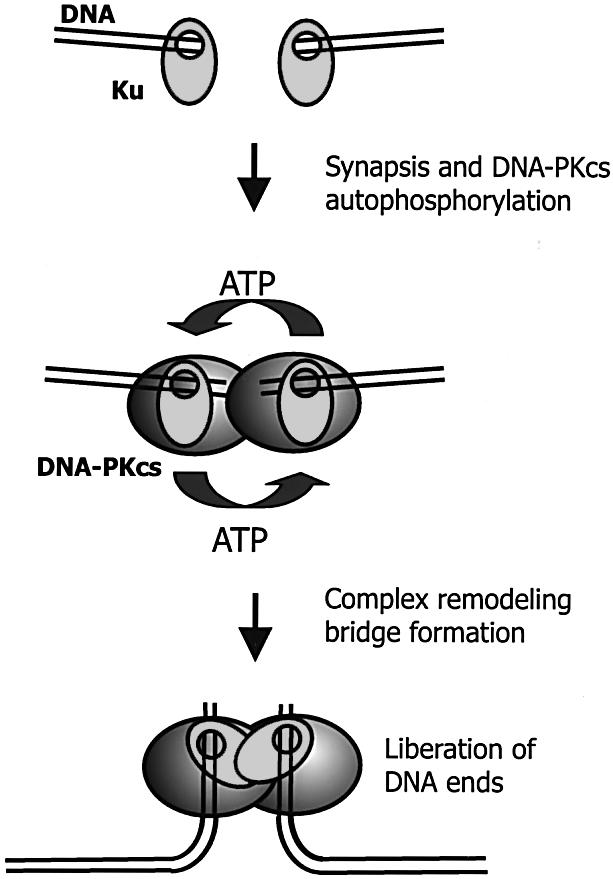
Model for DNA-PKCS autophosphorylation and its possible significance for in vivo end-joining. After the occurrence of a DSB, DNA-PK binds to the termini of both DNA fragments, thereby sterically protecting the termini against degradation by nucleases or (premature) ligation. Upon synapsis, DNA-PKCS autophosphorylation induces a (subtle) remodeling of the protein–DNA complex. This alteration of the end-bound complex makes the extreme ends accessible for ligases (or processing enzymes). Upon DNA-PKCS autophosphorylation, the termini are held together by the protein complexes, which function as a molecular bridge between the broken DNA fragments. Such a bridge would ensure stable association of both ends without blocking access to the extreme termini.
