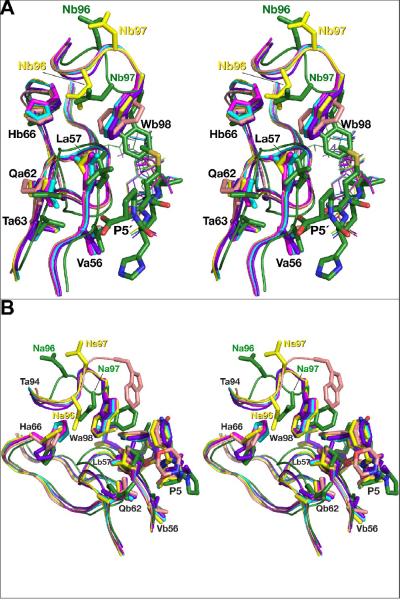
Figure 7.
Concerted conformational changes in the flaps and loops 91–99 induced by the interactions with the inhibitors. The structures of all complexes are superimposed on the basis of their Cα coordinates. AB dimers are used for the complexes with the statine-based inhibitor and KNI-10562. The flaps are shown as ribbons, fragments of loop 91–99 comprising residues 94–98 and the side chains of the flaps are shown in stick representation. The color scheme is the same as in Fig. 6. A) A view of the active sites of the enzymes interacting with the C-terminal half of the statine-based inhibitor (shown as sticks), with the KNI inhibitors shown in thin lines. B) Superimposed fragments of the active sites of the all inhibitor complexes interacting with the N-terminal halves of the inhibitors (shown as sticks).