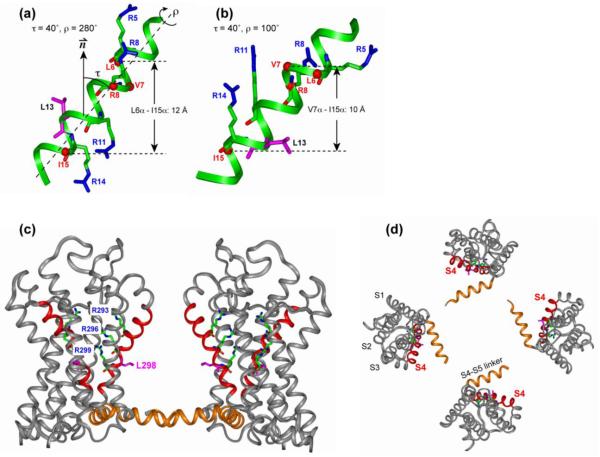
Figure 5.
Orientation of the S4 helix of Kv channels from NMR and crystal structures. (a, b) Solid-state NMR determined orientation of the isolated S4 helix in lipid bilayers. (a) τ = 40° and ρ = 280°. (b) τ = 40° and ρ = 100°. The four Arg sidechains and the Leu13 sidechain are shown as sticks. Cα atoms whose distances to lipid 31P were measured are shown as balls. (c, d) X-ray crystal structure of the voltage-sensing domain of the Kv2.1-Kv1.2 paddle-chimaera channel 6. (c) Side view with the pore axis oriented vertically. (d) Top view of the tetramer. The S4 helix is highlighted in red, and the S4-S5 linker is in orange. The three Arg residues corresponding to the second to fourth Arg’s in the NMR samples, and Leu298 (pink), corresponding to Leu13 of the NMR samples, are shown as sticks.