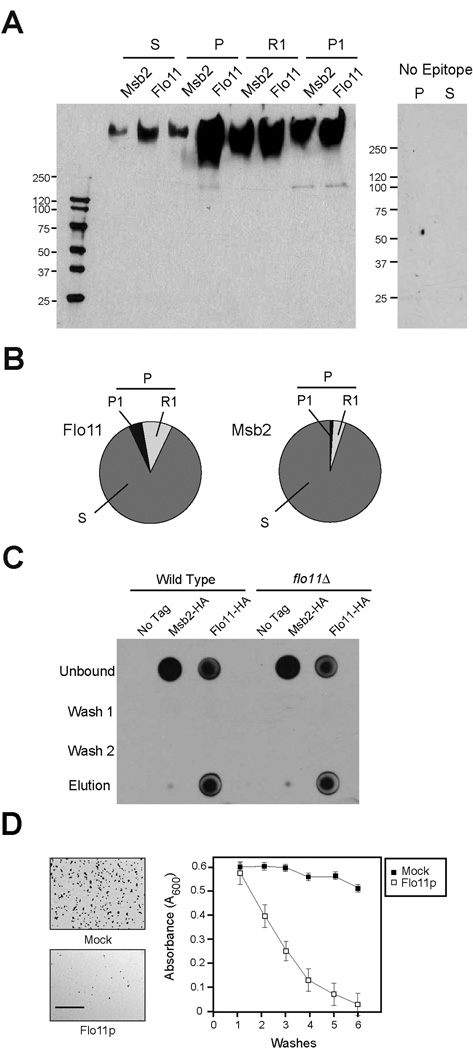
Figure 1. Properties of shed Flo11p.
A) Immunoblot analysis of supernatant (S) and pellet (P) fractions derived from cells expressing Flo11p-HA (PC2043) or Msb2p-HA (PC999). For panels A to C, strains were grown for 16 h in YEPD medium. Cells were concentrated by centrifugation and supernatants were used in immunoblotting with anti-HA antibodies or to test the properties of shed Flo11p. For panel A, the pellet fraction was further separated into R1 and P1 fractions by washing cell pellets in a neutral buffer (50 mM Tris pH 7) at 4° C for 30 min. At right, control S and P fractions from a non-tagged strain (PC538). B) Quantitation of shed (S), released (R1), and cell-associated (P, P1) Flo11p-HA and Msb2p-HA proteins. Band intensities in Fig. 1A were compared by densitometry and normalized to total input volumes (S, 10 mls, P, 200 µl, S1, 400 µl, P1, 100 µl). C) In vitro binding assay. Equal volumes of cells of untagged strains of wild type (PC538) and the flo11Δ (PC1029) mutant were incubated with equal volumes of conditioned media from wild type strains (non-tagged, PC538; Flo11p-HA, PC2043 and Msb2p-HA, PC999). After 2 h incubation at 30°C, cells were centrifuged and the supernatant fraction was used as the ‘Unbound’ fraction. Cells were washed twice with water (Wash 1 and 2) and treated with buffer (50mM Tris pH 9.5, 10mM DTT) to remove bound proteins. Eluates from this treatment along with washed and unbound fractions were spotted (50 µl) onto a nitrocellulose filter and examined by immunoblot analysis. D) Polystyrene surfaces were pre-coated with purified Flo11p (open squares) or water (Mock, filled squares). Cells were applied to pre-coated surfaces, which were sequentially washed with water (washes). Released cells were measured by absorbance at OD (A600) after each wash. The experiment was performed in duplicate; error bars represent the standard deviation between trials. At left, low magnification microscopic images showing cells adhering to surfaces pre-coated with water (upper panel) or purified shed Flo11p (lower panel). Bar = 500 microns.