Abstract
Neutrophil chemotaxis is a critical component in innate immunity. Recently, using a small-molecule functional screening, we identified NADPHoxidase- dependent Reactive Oxygen Species (ROS) as key regulators of neutrophil chemotactic migration. Neutrophils depleted of ROS form more frequent multiple pseudopodia and lost their directionality as they migrate up a chemoattractant concentration gradient. Here, we further studied the role of ROS in neutrophil chemotaxis and found that multiple pseudopodia formation induced by NADPH inhibitor diphenyleneiodonium chloride (DPI) was more prominent in relatively shallow chemoattractant gradient. It was reported that, in shallow chemoattractant gradients, new pseudopods are usually generated when existing ones bifurcate. Directional sensing is mediated by maintaining the most accurate existing pseudopod, and destroying pseudopods facing the wrong direction by actin depolymerization. We propose that NADPH-mediated ROS production may be critical for disruption of misoriented pseudopods in chemotaxing neutrophils. Thus, inhibition of ROS production will lead to formation of multiple pseudopodia.
Key words: chemokines, signal transduction, innate immunity, NADPH oxidase, chronic granulomatous disease, cell migration
Chemotaxis is a process in which cells sense and move up a gradient of molecules (chemoattractants). It plays a central role in the regulation of host defense and inflammatory reactions by recruiting circulating effector leukocytes, including neutrophils, monocytes and effector T cells to the sites of injury or infection. Neutrophil chemotaxis is mediated by heterotrimeric guanine nucleotide-binding regulatory proteins (G proteins) coupled receptors (GPCR). Chemoattractants bind membrane receptors and initiate accumulation of PtdIns(3,4,5)P3 and subsequent actin polymerization at the leading edge of chemotaxing cells. Earlier studies have suggested that PtdIns(3,4,5)P3 plays essential role of a cellular compass.1–4 However, several recent studies have shown that loss of PI3K and reduced PtdIns(3,4,5) P3 level lead to decreased polarity, but does not affect the ability of the cell to sense chemoattractant gradients5,6 and Dictyostelium,7–9 suggesting extra pathways are required for neutrophil chemotaxis. Similarly, enhancing PtdIns(3,4,5) P3 signal only augments the sensitivity of neutrophils to chemoattractant stimulation. 10–13 In an attempt to identify the extra putative signal-induced chemotactic pathways, we conducted a functional screening for chemical compounds that disrupt neutrophil directionality. We identified NADPH oxidase-dependent Reactive Oxygen Species (ROS) as key regulators of neutrophil chemotaxis.14
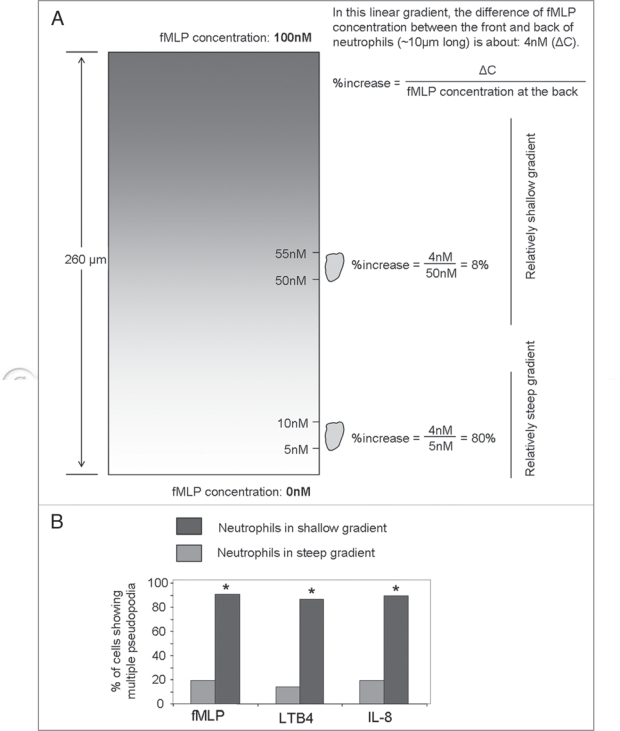
During chemotaxis, chemoattractants elicit a number of changes in neutrophils. These include a localized polymerization of F-actin at the site of cell cortex closest to the chemoattractant source, a morphological change characterized by cell elongation, the formation of new lamellipodia or pseudopods at the leading edge, and the forward protrusion of the leading edge followed by retraction of posterior of the cell. We found that neutrophils with inhibited ROS production, that were isolated from CGD patients/mice or pharmacologically/ siRNA treated to inhibit the NADPH oxidase complex, formed more frequent multiple pseudopodia and lost their directionality as they migrated up a chemoattractant gradient.14 The functional screening was performed using an EZ-Taxiscan chemotaxis device in which a stable chemoattractant gradient was formed in a 260 µm-wide channel.14 Interestingly, the most dramatic multiple pseudopodia formation induced by NADPH inhibitor diphenyleneiodonium chloride (DPI) was observed in the middle part of the device, where the chemoattractant gradient was relatively shallow (Fig. 1A). It is noteworthy that ROS does not appear to be involved in directional sensing per se, since most neutrophils depleted of ROS can still migrate up the chemoattractant gradient. At the lower section of the channel, where the chemoattractant gradient was relatively steep, the DPIinduced multiple pseudopodia formation was less prominent (Fig. 1B). This result suggested that the dependent on ROS in neutrophil chemotaxis may rely on the feature of the gradient.
Figure 1.
Multiple pseudopodia formation induced by nadPH inhibitor diphenyleneiodonium chloride (dPI) was more prominent in relatively shallow chemoattractant gradient. (a) relatively shallow chemoattractant gradient was generated in the upper section in the eZ-taxiscan device. (B) dependent on rOS in neutrophil chemotaxis relies on the feature of the gradient. neutrophils were treated with 50 µM dPI for 30 min and chemotaxis was induced by 100 nM fMLP. neutrophil purification, eZ-taxiscan chemotaxis assay, and analysis of cell tracks and morphology were conducted as previously described.14,18,19 Percentage of cells that display multiple pseudopodia (n = 20 cells, Fisher’s 2 × 2 test, *p < 0.05 versus untreated) during the course of the eZ-taxiscan chemotaxis assay was quantified as described by Hattori et al.14
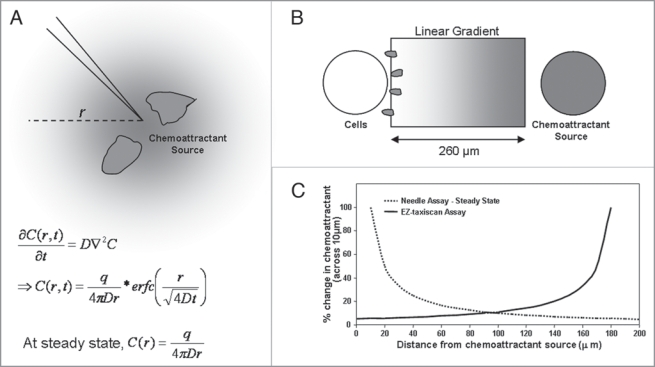
To further confirm that ROS is dispensable for neutrophil chemotaxis in steep gradient of chemoattractant, we generated the gradient using a micropipette. In this setup, a micropipette (Eppendorf Femtotip with an opening of 0.5 µm) filled with 10 µM fMLP was lowered onto a cover slip plated with neutrophils. Chemoattractant gradient was formed by continuous passive diffusion from the tip of the micropipette. It was well documented that the chemoattractant gradient generated by this device is steepest near the source (Fig. 2A–C). We examined chemotactic behavior of cells within a 50 µm radius. We observed stable formation of single pseudopodia in both untreated and DPI-treated neutrophils, again suggesting that multiple pseudopod formation induced by ROS depletion was less prominent in relatively steep chemoattractant gradient.
Figure 2.
Comparison of various chemotaxis assays. (a) needle assay. Chemoattractant gradient was formed by continuous passive diffusion of chemoattractant from a micropipette tip. equations describe the concentration gradient C(r,t) generated in the radial direction (neglecting convection).20 D denotes the diffusion constant for the chemoattractant (cm2/sec), q denotes the rate at which the chemoattractant is released (mols/sec), r is the radius from the needle tip (cm). (B) eZ-taxis scan chemotaxis device. Gradient is set up by addition of 1 µl chemoattractant to the chemoattractant reservoir, and allowing diffusion towards the cell reservoir. a linear gradient is setup across a 260 µm channel within the time scale of the experiment (30 mins) (as per manufacturer’s description). (C) Comparison of gradients generated by needle assay and eZ-taxiscan device. Percentage change in chemoattractant concentration across 10 µm sections are plotted for the steady state gradient in the needle assay (C ∼1/r) and a linear gradient in a eZ-taxiscan device. the chemoattractant gradient is most shallow near the source for the eZ-taxiscan assay and steepest near the source for the needle assay.
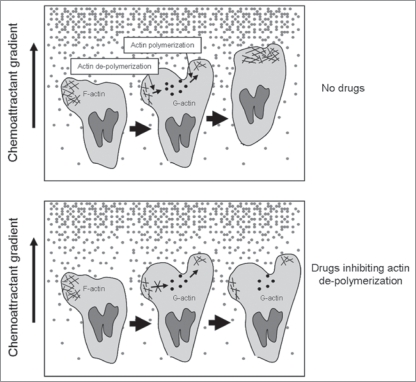
Based on these results, we propose that ROS may only be involved in regulating pseudopod formation in neutrophils exposed to shallow chemoattractant gradient. It was recently reported that, in shallow chemoattractant gradients, new pseudopods are usually generated when existing ones bifurcate.6 The location and direction of pseudopod formation are thought to be random and are not oriented by chemoattractants. Directional sensing is mediated by maintaining the most accurate existing pseudopod, and destroying pseudopods facing the wrong direction by actin depolymerization. NADPH mediated ROS production may be critical for disruption of misoriented pseudopods in chemotaxing neutrophils. Thus, inhibition of ROS production will lead to formation of multiple pseudopodia (Fig. 3).
Figure 3.
neutrophil chemotaxis in shallow chemoattractant gradient. In shallow chemoattractant gradients, new pseudopods are usually generated when existing ones bifurcate. their location and direction are random and are not oriented by chemoattractants. directional sensing is mediated by maintaining the most accurate existing pseudopod, while the ones facing wrong direction need to be quickly destroyed via actin depolymerization. Inhibition of actin depolymerization in misoriented pseudopods should lead to multiple pseudopod formation and reduced chemotaxis efficiency.
The mechanism by which ROS regulates pseudopod formation remains elusive. ROS can oxidize thiols (-SH) on protein cysteine residues, leading to reversible protein post-translational modifications such as glutathionylation, disulfide bond formation and sulfenic acid formation. Redox regulation of numerous signaling proteins such as Ras, protein tyrosine kinases (Src kinases), and protein tyrosine phosphatases, have been reported.15–17 These modifications often alter functionality/activity of the targeted proteins.15–17 ROS can also regulate actin polymerization via modifying G-actin monomers.15–17 Thus, they may directly affect actin polymerization/ depolymerization in chemotaxing neutrophils.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/11559
References
- 1.Iijima M, Devreotes P. Tumor suppressor PTEN mediates sensing of chemoattractant gradients. Cell. 2002;109:599–610. doi: 10.1016/s0092-8674(02)00745-6. [DOI] [PubMed] [Google Scholar]
- 2.Funamoto S, Meili R, Lee S, Parry L, Firtel RA. Spatial and temporal regulation of 3-phosphoinositides by PI3-kinase and PTEN mediates chemotaxis. Cell. 2002;109:611–623. doi: 10.1016/s0092-8674(02)00755-9. [DOI] [PubMed] [Google Scholar]
- 3.Li Z, Dong X, Wang Z, Liu W, Deng N, Ding Y, et al. Regulation of PTEN by Rho small GTPases. Nat Cell Biol. 2005;7:399–404. doi: 10.1038/ncb1236. [DOI] [PubMed] [Google Scholar]
- 4.Li Z, Hannigan M, Mo Z, Liu B, Lu W, Wu Y, et al. Directional sensing requires Gbetagamma-mediated PAK1 and PIXalpha-dependent activation of Cdc42. Cell. 2003;114:215–227. doi: 10.1016/s0092-8674(03)00559-2. [DOI] [PubMed] [Google Scholar]
- 5.Chodniewicz D, Zhelev DV. Chemoattractant receptor- stimulated F-actin polymerization in the human neutrophil is signaled by 2 distinct pathways. Blood. 2003;101:1181–1184. doi: 10.1182/blood-2002-05-1435. [DOI] [PubMed] [Google Scholar]
- 6.Andrew N, Insall RH. Chemotaxis in shallow gradients is mediated independently of PtdIns 3-kinase by biased choices between random protrusions. Nat Cell Biol. 2007;9:193–200. doi: 10.1038/ncb1536. [DOI] [PubMed] [Google Scholar]
- 7.Chen L, Janetopoulos C, Huang YE, Iijima M, Borleis J, Devreotes PN. Two phases of actin polymerization display different dependencies on PI(3,4,5) P3 accumulation and have unique roles during chemotaxis. Mol Biol Cell. 2003;14:5028–5037. doi: 10.1091/mbc.E03-05-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loovers HM, Postma M, Keizer-Gunnink I, Huang YE, Devreotes PN, van Haastert PJ. Distinct roles of PI(3,4,5)P3 during chemoattractant signaling in Dictyostelium: a quantitative in vivo analysis by inhibition of PI3-kinase. Mol Biol Cell. 2006;17:1503–1513. doi: 10.1091/mbc.E05-09-0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen L, Iijima M, Tang M, Landree MA, Huang YE, Xiong Y, et al. PLA2 and PI3K/PTEN pathways act in parallel to mediate chemotaxis. Dev Cell. 2007;12:603–614. doi: 10.1016/j.devcel.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Subramanian KK, Jia Y, Zhu D, Simms BT, Jo H, Hattori H, et al. Tumor suppressor PTEN is a physiologic suppressor of chemoattractant-mediated neutrophil functions. Blood. 2007;109:4028–4037. doi: 10.1182/blood-2006-10-055319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jia Y, Subramanian KK, Erneux C, Pouillon V, Hattori H, Jo H, et al. Inositol 1,3,4,5-tetrakisphosphate negatively regulates phosphatidylinositol-3,4,5- trisphosphate signaling in neutrophils. Immunity. 2007;27:453–467. doi: 10.1016/j.immuni.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarraj B, Massberg S, Li Y, Kasorn A, Subramanian K, Loison F, et al. Myeloid-specific deletion of tumor suppressor PTEN augments neutrophil transendothelial migration during inflammation. J Immunol. 2009;182:7190–7200. doi: 10.4049/jimmunol.0802562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y, Jia Y, Pichavant M, Loison F, Sarraj B, Kasorn A, et al. Targeted deletion of tumor suppressor PTEN augments neutrophil function and enhances host defense in neutropenia-associated pneumonia. Blood. 2009;113:4930–4941. doi: 10.1182/blood-2008-06-161414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hattori H, Subramanian KK, Sakai J, Jia Y, Li Y, Porter TF, et al. Small-molecule screen identifies reactive oxygen species as key regulators of neutrophil chemotaxis. Proc Natl Acad Sci USA. 2010 doi: 10.1073/pnas.0914351107. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 16.Clavreul N, Bachschmid MM, Hou X, Shi C, Idrizovic A, Ido Y, et al. S-glutathiolation of p21ras by peroxynitrite mediates endothelial insulin resistance caused by oxidized low-density lipoprotein. Arterioscler Thromb Vasc Biol. 2006;26:2454–2461. doi: 10.1161/01.ATV.0000242791.28953.4c. [DOI] [PubMed] [Google Scholar]
- 17.Shelton MD, Chock PB, Mieyal JJ. Glutaredoxin: role in reversible protein s-glutathionylation and regulation of redox signal transduction and protein translocation. Antioxid Redox Signal. 2005;7:348–366. doi: 10.1089/ars.2005.7.348. [DOI] [PubMed] [Google Scholar]
- 18.Zhu D, Hattori H, Jo H, Jia Y, Subramanian KK, Loison F, et al. Deactivation of phosphatidylinositol 3,4,5-trisphosphate/Akt signaling mediates neutrophil spontaneous death. Proc Natl Acad Sci USA. 2006;103:14836–14841. doi: 10.1073/pnas.0605722103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kasorn A, Alcaide P, Jia Y, Subramanian KK, Sarraj B, Li Y, et al. Focal adhesion kinase regulates pathogen- killing capability and life span of neutrophils via mediating both adhesion-dependent and -independent cellular signals. J Immunol. 2009 doi: 10.4049/jimmunol.0802984. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodhill GJ. Diffusion in axon guidance. Eur J Neurosci. 1997;9:1414–1421. doi: 10.1111/j.1460-9568.1997.tb01496.x. [DOI] [PubMed] [Google Scholar]