Fig. 1.
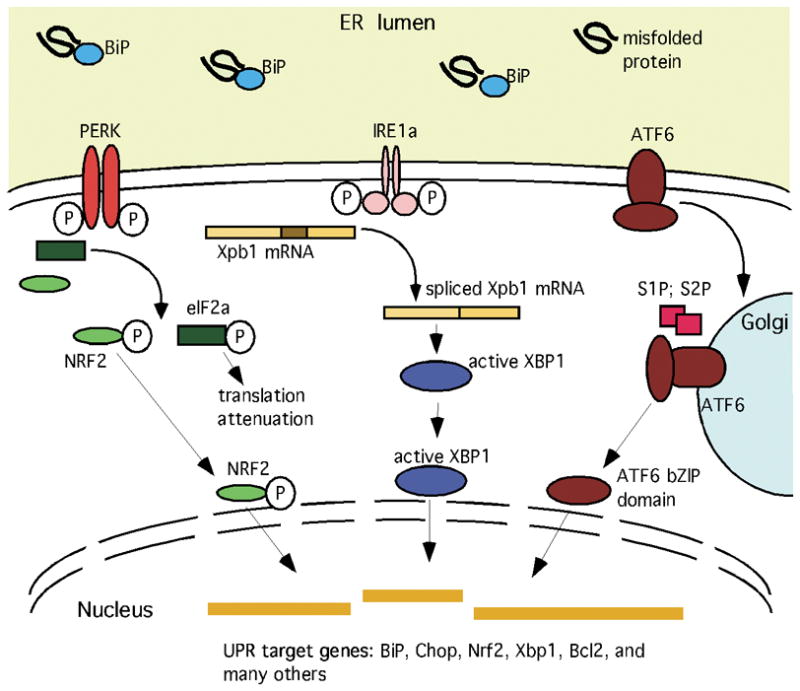
Schematic depiction of selected core elements of the unfolded protein response (UPR), featuring the components measured herein (Schaefer et al., 2001; Schroder and Kaufman, 2005; Zhang and Kaufman, 2008a; b). Accumulation of unfolded proteins in the ER lumen competitively reduces BiP binding to PERK, IRE1a, and ATF6, releasing them to carry out their cytoplasmic and nuclear activities. PERK phosphorylates several substrates, including NRF2 and EIF2a. The latter event decreases translation of most mRNAs, but also increases translation of a few others, such as the transcription factor Atf4 (not depicted). IRE1a dimerizes, activating its endoribonuclease activity and thereby reducing Xbp1 mRNA into a transcript that encodes an active bZIP transcription factor. ATF6 translocates to the Golgi apparatus where the action of two proteases (S1P and S2P) release a bZIP transcription factor domain. The increased availability of these transcriptional regulators alters expression of many genes, including the core elements of the URP pathway itself, molecular chaperones, proteins involved in the degradation of ER proteins, antioxidant proteins, metabolic enzymes, regulators of the cell cycle, and survival/apoptotic factors.
