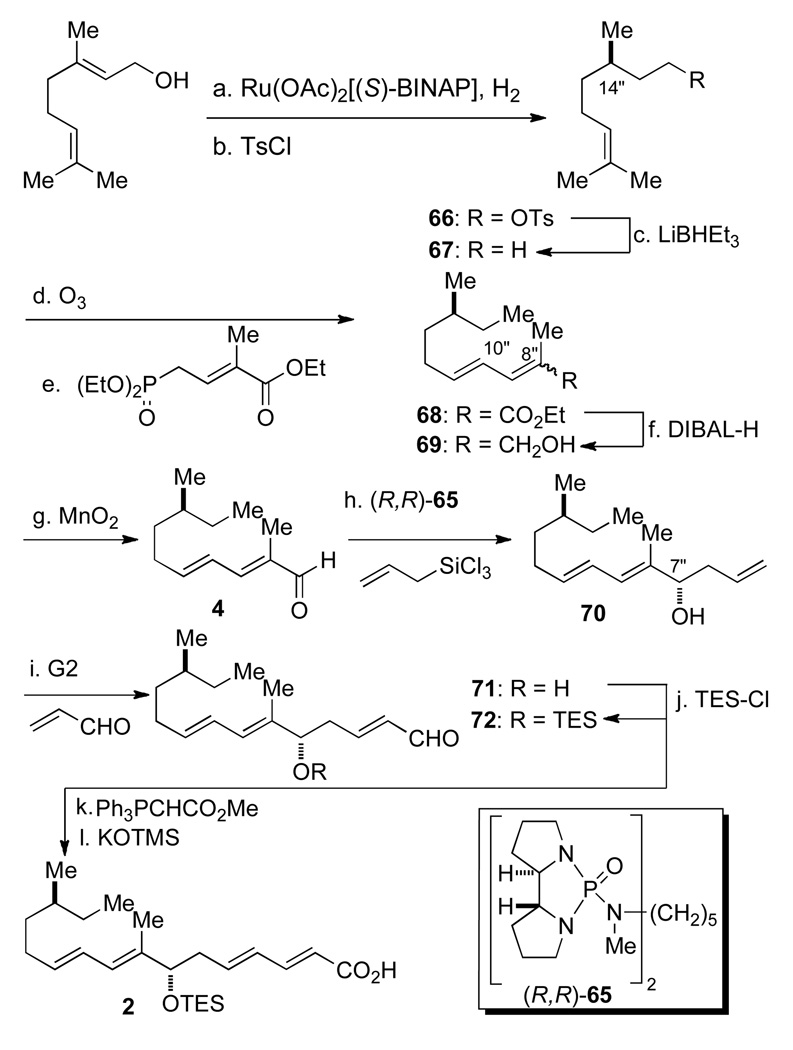
Scheme 16a.
aConditions: (a) Ru(OAc)2[(S)-BINAP] (0.7 mol %), H2 (1500 psi), 95% MeOH, rt, 20 h, 99% (97:3 er); (b) Ts-Cl, pyridine, rt, 10 h, 89%; (c) LiHBEt3, THF, NaOH-H2O2, rt, 1 h, 86%; (d) O3, NaHCO3, CH2Cl2-MeOH, −78 °C to rt, DMS, 2.5 h; (e) (EtO)2POCH2C=C(CH3)CO2Et, LiOH, MS 4Å, THF, reflux, 2 h, 78% (2 steps) (E,E : E,Z 91:9); (f) DIBAL-H, THF, 0 °C, 0.5 h, 85% (pure E,E); (g) MnO2, CHCl3, reflux, 4 h, 89%; (h) (R,R)-65 (10 mol%), allyltrichlorosilane, CH2Cl2, i-Pr2EtN, −78 °C, 8 h, 88% (96:4 dr); (i) Grubb’s 2nd gen. catalysts (5.0 mol %), acrolein (13 equiv), CH2Cl2, 90%; (j) TES-Cl, 2,6-lutidine, CH2Cl2, rt, 4 h, 92%; Ph3P=CCO2Me, ClCH2CH2Cl, reflux, 18 h, 90% (E:Z 90:10); (k) TMSOK, THF, rt, 4 h, 94%.