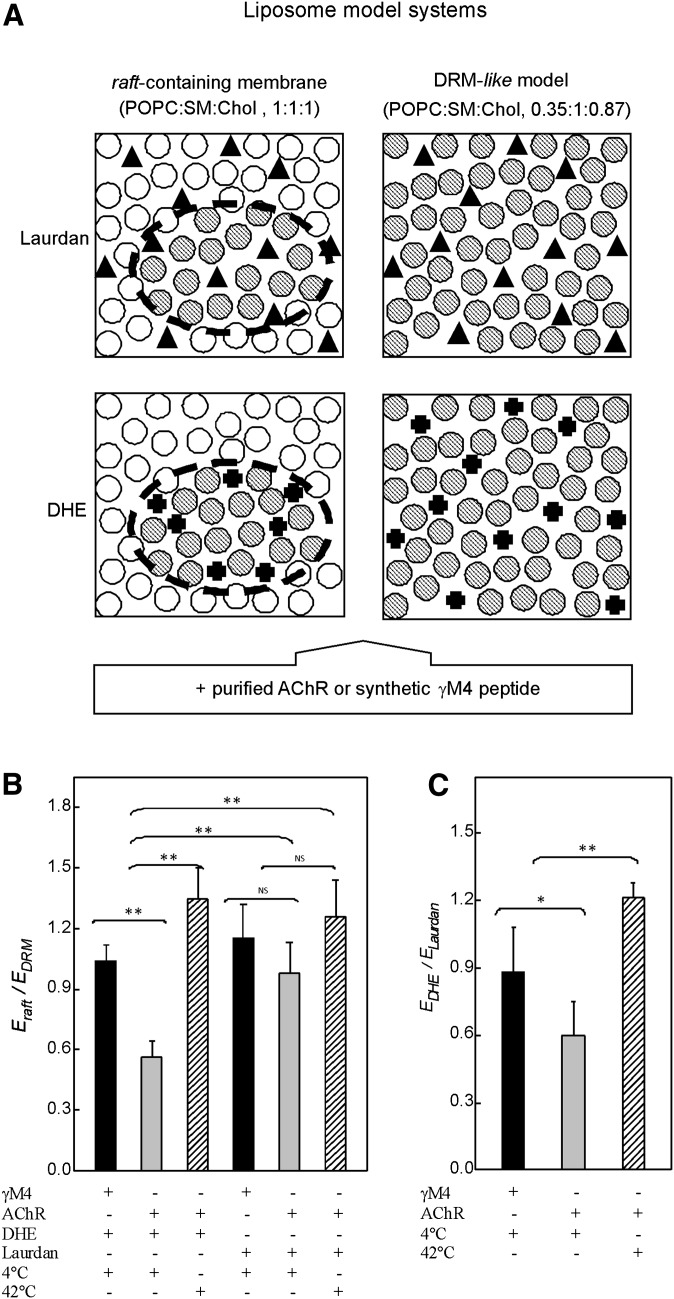
Fig. 5.
A: Lipid model systems used in FRET studies: raft-containing membrane (POPC:SM:Chol, 1:1:1 molar ratio), where there is a coexistence of two different lipid domains (⊘, ○ for DRM and DSM-like domains, respectively), and DRM-like model (POPC:SM:Chol, 0.35:1:0.87 molar ratio) consisting of only one lipid domain (⊘). Laurdan fluorescent probe (▴), used as a control probe, shows no preferential partition into a particular domain. DHE (✚), used as a fluorescent Chol mimetic, labels DRM domains. FRET measurements were performed between the intrinsic fluorescence of the γM4 peptide or the AChR, donor molecules, and Laurdan or DHE as acceptor probes. B: Ratio of the FRET efficiencies between the intrinsic fluorescence of the purified AChR (or the γM4 peptide) as donor molecules and DHE (or Laurdan) as acceptor molecules obtained with the raft-containing membrane (Eraft) and with the DRM-like model (EDRM) at 4°C and 42°C. C: Ratio of the FRET efficiencies obtained between the intrinsic fluorescence of the purified AChR (or the γM4 peptide) as donor molecules and DHE (EDHE) and Laurdan (ELaurdan) as acceptor molecules in the raft-containing membrane at 4°C and 42°C. Each column corresponds to the average ± SD of at least four independent measurements. *, ** Statistically significant differences: *P < 0.05; **P < 0.01. NS (nonsignificant) differences: P < 0.05. AChR, nicotinic acetylcholine receptor; Chol, cholesterol; DHE, dehydroergosterol; DRM, detergent-resistant membrane; DSM, detergent-soluble membrane; E, energy transfer efficiency; FRET, Förster resonance energy transfer; POPC, palmitoyloleylphosphatidilcholine; SM, brain sphingomyelin.