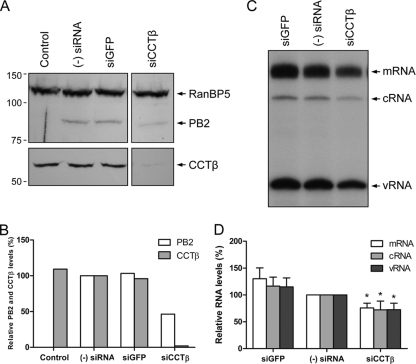
FIG. 5.
The effect of CCT knockdown on PB2 protein and viral RNA levels. (A) Western blot analysis of PB2 and CCTβ in untreated 293T cells [(Control and (−) siRNA] or 293T cells treated with GFP- or CCTβ-specific siRNA (siGFP or siCCTβ, respectively). Cells were also transfected with pcDNA-PB1, pcDNA-PB2, pcDNA-PA, pcDNA-NP, and pPOLI-NA-RT plasmids to express recombinant ribonucleoproteins. No plasmids were transfected in the control cells. PB2 was detected with a polyclonal PB2-specific antibody (3). RanBP5 detected with a polyclonal RanBP5-specific antibody (Santa Cruz) was used as a loading control. Positions of molecular mass markers (Bio-Rad) in kDa are indicated on the left. (B) Quantitation of Western blot analysis of PB2 and CCTβ protein levels from panel A. PB2 and CCTβ intensities in siRNA-treated cells were expressed as a percentage of intensities observed in cells not treated with siRNA (−), which was set to 100%. PB2 and CCTβ levels were normalized to the levels of RanBP5. (C) Primer extension analysis of mRNA, cRNA, and vRNA of the neuraminidase gene in CCTβ-silenced 293T cells. Cells were treated with siRNAs, followed by the transfection of plasmids to reconstitute ribonucleoprotein complexes as in panel A. (D) Quantitation of primer extension analysis of viral mRNA, cRNA, and vRNA levels from panel C. RNA levels in siRNA-treated cells were expressed as a percentage of RNA levels observed in untreated cells [(−) siRNA], which was set to 100%. RNA levels were normalized to the total amount of RNA used in the primer extension assay. Bars represent standard deviations based on four independent experiments. *, P < 0.05, based on one-sample Student's t test.