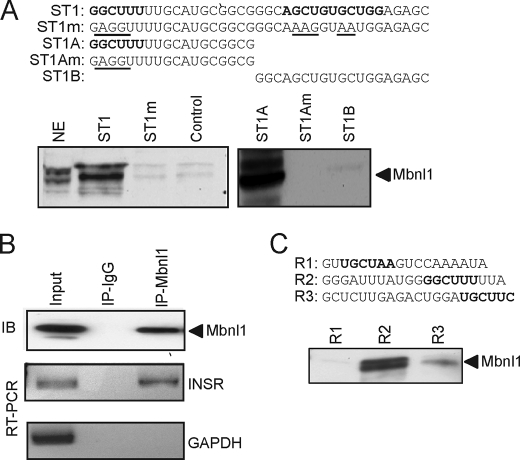
FIGURE 5.
Mbnl1 binds to the insulin receptor RNA both in vitro and in vivo. A, sequences of the RNA oligonucleotides, derived from human intron 11, used for RNA affinity purification. Putative Mbnl1-binding sites are marked in boldface, and mutations are underlined. RNA oligonucleotides were covalently linked to adipic acid dihydrazide-agarose beads. HeLa nuclear extracts were incubated with the beads and washed extensively, and then associated proteins were eluted with SDS-PAGE sample buffer. Bound proteins were separated on 4–12% BisTris gels and immunoblotted with anti-Mbnl1. NE indicates input HeLa nuclear extract (1/25th of input). Representative blots are shown. Experiment was repeated five times with similar results. B, intracellular association between Mbnl1 and the endogenous INSR transcript was analyzed by ribonucleoprotein immunoprecipitation. Endogenous Mbnl1 was immunoprecipitated (IP). Immunoblot (IB) shows that Mbnl1 protein is immunoprecipitated by anti-Mbnl1 antibody but not by mouse IgG (upper panel). RT-PCR assay shows that IR mRNA is coimmunoprecipitated by anti-Mbnl1 antibodies but not by IgG (middle panel). RT-PCR assay for control glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA (lower panel) is shown. C, sequences of the RNA oligonucleotides derived from rat intron 11 used for RNA affinity purification. Putative Mbnl1-binding sites are marked boldface. Representative Western blot for Mbnl1 is shown.