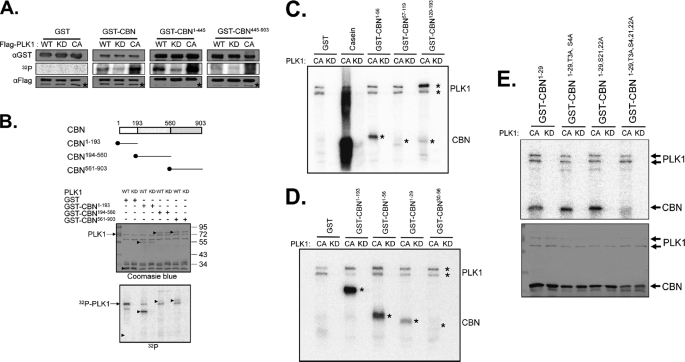
FIGURE 3.
In vitro kinase assay of centrobin by PLK1. A, WT, KD, or CA forms of the FLAG-PLK1 proteins were ectopically expressed in 293T cells and immunoprecipitated with the FLAG antibody. Bacterially expressed centrobin fusion proteins (GST, GST-CBN, GST-CBN1–445, and GST-CBN445–903) were used as substrates. Amounts of the enzymes and substrates were determined with immunoblot analyses and the kinase activity was determined via autoradiography. Asterisks in the immunoblot data indicate the IgG heavy chain bands. B, the in vitro kinase assay was performed with the WT and KD FLAG-PLK1 proteins. As substrates, bacterially expressed GST, GST-CBN1–193, GST-CBN194–560, and GST-CBN561–903 were used. Arrowheads indicate the GST fusion proteins. C–E, the in vitro kinase assay was performed with the bacterially expressed kinase-active (CA) or kinase-dead (KD) PLK1 protein. C, as substrates, GST-CBN1–193 was truncated into three fragments (GST-CBN1–56, GST-CBN57–119, and GST-CBN120–193). Casein was used as a control substrate. D, as substrates, GST-CBN1–56 was truncated into two fragments (GST-CBN1–29 and GST-CBN30–56). The autophosphorylated PLK1 and phosphorylated GST-CBN proteins were marked with asterisks. E, the GST-CBN1–29 fusion proteins in which 4 specific serine or threonine residues were mutated into alanines (GST-CBN1–29, GST-CBN1–29,T3A,S4A, GST-CBN1–29,S21,22A, GST-CBN1–29,T3A,S4,21,22A) were used as substrates. The phosphorylation activity was determined by autoradiography, and the amount of the substrates was determined by Coomassie Blue staining.