FIGURE 3.
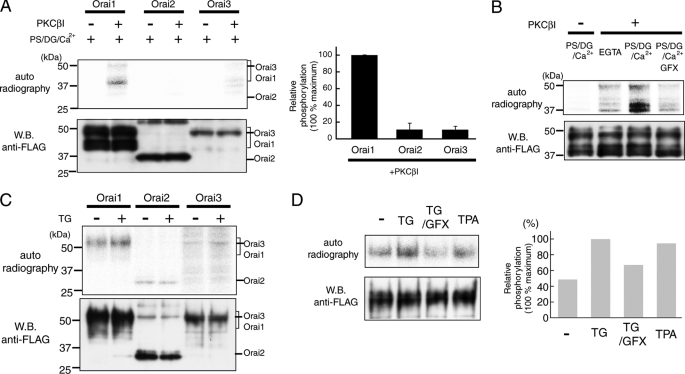
Orai1 is phosphorylated by PKCβI in vitro and in vivo. A and B, in vitro phosphorylation of Orai1. A, FLAG-tagged Orai proteins (Orai1, -2, and -3) were purified and incubated with or without recombinant PKCβI in the presence of PKC activator (PS/DG/Ca2+) and [γ-32P]ATP. Phosphorylated proteins were detected by autoradiography, and protein expression was determined by Western blotting using anti-FLAG antibody. The bar graph shows relative phosphorylation levels normalized to the amount of total protein (n = 3). B, purified Orai1 was incubated with PKCβI and PS/DG/Ca2+ in the presence or absence of PKC inhibitor GFX (5 μm). C and D, in vivo phosphorylation of Orai1. C, HEK293 cells expressing FLAG-tagged Orai proteins (Orai1, -2, and -3) were incubated with 32P-monosodium phosphate and stimulated with TG for 120 s in the presence of 2 mm extracellular Ca2+. Phosphorylated proteins were detected by autoradiography. D, cells expressing FLAG-tagged Orai1 were stimulated with TG in the presence or absence of GFX (1 μm), and with TPA (500 nm). Relative phosphorylation levels for each experimental condition were normalized to the strongest phosphorylation signal following TG stimulation (set as 100%). Averages of Orai1 phosphorylation from two independent experiments are shown.