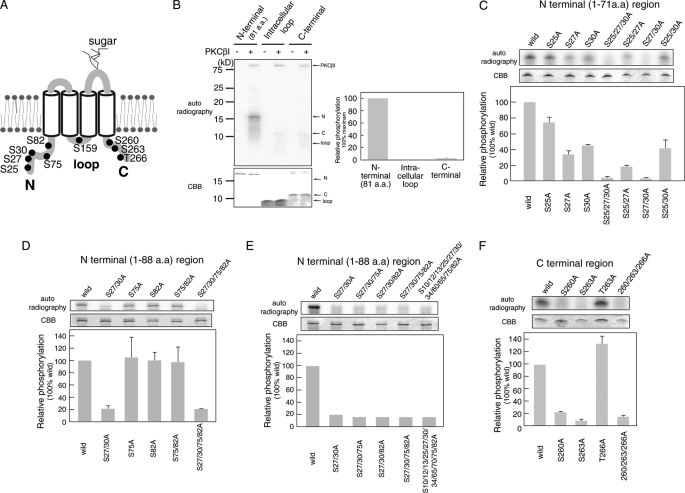
FIGURE 4.
Analysis of PKC phosphorylation sites in intracellular regions of Orai1. A, the predicted PKC phosphorylation sites in Orai1 are shown. B–F, in vitro phosphorylation of the Orai1 N terminus (aa 1–81), intracellular loop (aa 141–173), and C terminus (aa 257–301). Intracellular Orai1 protein regions were expressed in E. coli, purified, and incubated with [γ-32P]ATP and recombinant PKCβI. Phosphorylated proteins were separated by SDS-PAGE and detected by autoradiography. Expression levels of recombinant Orai1 protein regions were detected by Coomassie Brilliant Blue (CBB) staining. Densitometry results of autoradiographs are shown in the bar graphs. Phosphorylation levels were normalized to the strongest phosphorylation signal (B) or phosphorylation of wild-type Orai1 (C–F), which were set to 100%. B, in vitro phosphorylation of the Orai1 N terminus (80 pmol), intracellular loop (400 pmol), and C terminus (240 pmol). C–F, in vitro phosphorylation of the Orai1 N terminus, intracellular loop, and C terminus containing the indicated mutations of predicted serine phosphorylation sites. Bars represent mean ± S.D. from three independent experiments.