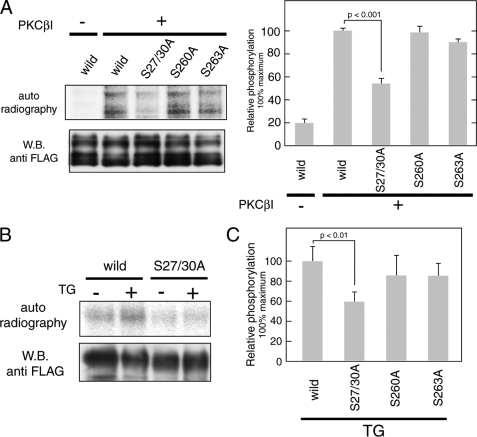
FIGURE 5.
PKC mediates phosphorylation of full-length Orai1 protein at Ser-27 and Ser-30. A, in vitro kinase assay using full-length Orai1. FLAG-tagged wild-type and mutant (S27A/S30A, S260A, and S263A) Orai1 was expressed in COS7 cells and purified by anti-FLAG-agarose resin. Full-length proteins were incubated with [γ-32P]ATP and recombinant PKCβI. Phosphorylated proteins were separated by SDS-PAGE and detected by autoradiography. Total amounts of protein were determined by Western blotting using anti-FLAG antibody. Bar graphs represent the averages of densitometry results ±S.D. (n = 3); phosphorylation levels for wild-type Orai1 in the presence of PKCβI were set to 100%. B, in vivo phosphorylation of Orai1 in HEK293 cells. Cells transfected with FLAG-tagged wild-type and S27A/S30A Orai1 mutants were left unstimulated or stimulated with TG after incubation with 32P-monosodium phosphate. FLAG-tagged Orai1 was precipitated and separated by SDS-PAGE. Phosphorylated Orai proteins were detected by autoradiography. Total amounts of protein were determined by Western blotting using anti-FLAG antibody. C, phosphorylation levels of FLAG-tagged Orai1 mutants (S27A/S30A, S260A, and S263A) after TG stimulation were normalized to phosphorylation of wild-type Orai1 (set to 100%). Bars represent averages ± S.D. (n = 3).