Figure 4.
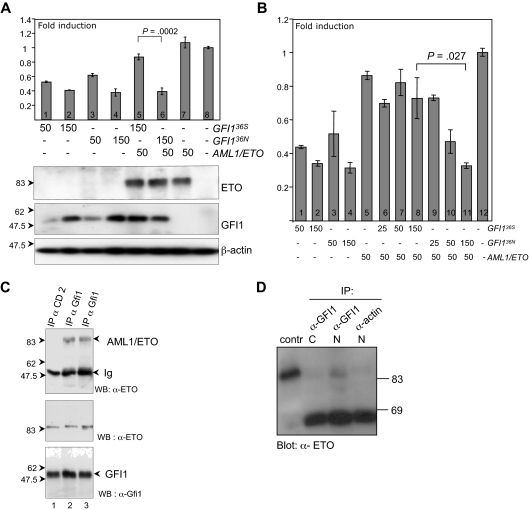
GFI136S and GFI136N maintain similar repressor activity but are differentially regulated by AML1/ETO. (A) Transient transfection of HeLa cells with expression plasmids for GFI136S, GFI136N, AML1/ETO fusion protein, and with a luciferase reporter gene containing Gfi1 binding sequences. Both GFI136S and GFI136N were able to repress the Gfi1 promoter in a concentration-dependent manner (lanes 1–2 and 3–4, respectively), whereas AML1/ETO alone did not influence reporter gene activity (lane 8). In the presence of AML1/ETO, the GFI136S protein lost its repression capacity, but GFI136N retained its repressor activity (lanes 5–6; P < .001). Error bars represent SEM. (B) Same as panel A but with different amounts of expression plasmid transfected as indicated. (C) GFI136S and GFI136N both coprecipitate in transfected cells with AML1/ETO. Cos7 cells were transfected with GFI136S, GFI136N, or AML1/ETO expression plasmids. Immunoprecipitations (IP) were performed with α-GFI1 antibody. AML1/ETO immunoprecipitates in the lysates with GFI136S or the variant GFI136N (lanes 2 and 3, respectively). A control IP was performed with a goat α-CD2 antibody (lane 1). An input control demonstrated the presence of both GFI1 and the AML1/ETO protein. (D) GFI136S coprecipitates with AML1/ETO in extracts from Kasumi1 cells, which is a cell line derived from a t(8;21) AML patient. Nuclear and cytosolic extracts of Kasumi1 cells were prepared and incubated either with the α-Gfi1 (N20) antibody or with a nonspecific α-actin antibody. The cell extracts were immunoblotted and developed with an α-ETO antibody. As a positive control, AML1/ETO transfected Cos7 cells were used.