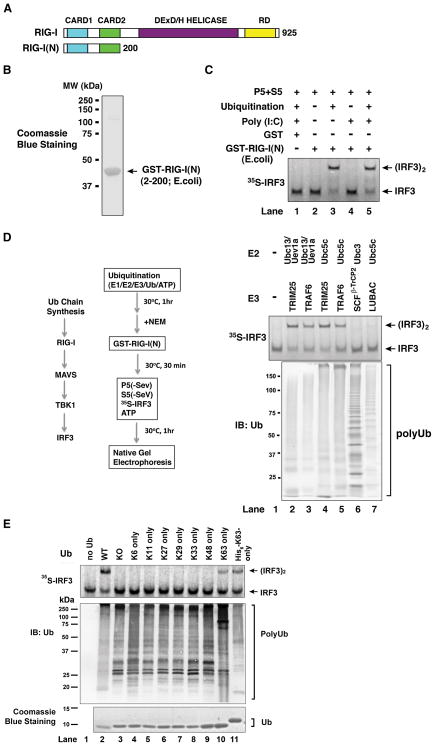
Figure 3. Activation of RIG-I N-terminus by K63 Polyubiquitin Chains.
(A) Depiction of RIG-I functional domains. (B) Purification of recombinant GST-RIG-I(N) protein from E. coli. (C) Activation of RIG-I(N) by ubiquitination. GST or GST-RIG-I(N) was incubated with ubiquitination reaction (E1, Ubc5, TRIM25 and ubiquitin) and/or poly(I:C) as indicated, then an aliquot of the reaction was further incubated with mitochondria (P5) and cytosol (S5) to measure IRF3 dimerization. (D) K63 polyUb chain synthesis, but not ubiquitination of RIG-I(N), is required for RIG-I activation in vitro. Ubiquitination reactions containing different combinations of E2s and E3s as indicated were carried out before N-ethylmaleimide (NEM) was added to inactivate E1 and E2s. An aliquot of the reaction mixture was incubated with GST-RIG-I(N), then further incubated with mitochondria (P5) and cytosol (S5) to measure IRF3 dimerization. Another aliquot of the reaction was analyzed by immunoblotting with a ubiquitin antibody (lower panel). SCFβ-TrCP2: an E3 complex containing Skp1, Cul1, Rbx1 and β-TrCP2; LUBAC: an E3 complex containing HOIL-1L and HOIP. (E) Similar to D, except that ubiquitination reactions were carried out in the presence of Ubc5c, TRIM25 and various ubiquitin mutants as indicated. See also Figure S3.