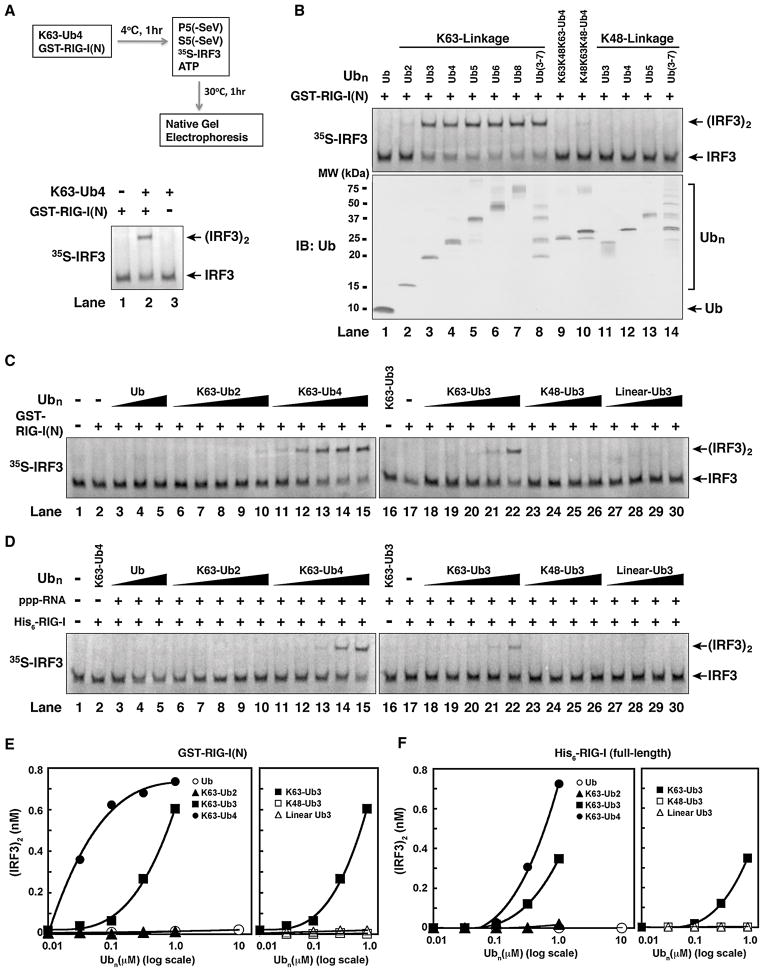
Figure 4. Short, Unanchored, K63-Ubiquitin Chains Potently Activate RIG-I.
(A) K63-Ub4 activates RIG-I(N). GST-RIG-I(N) (0.2 μM) was incubated with or without K63-Ub4 (0.3 μM), followed by IRF3 dimerization assay as outlined. (B) K63, but not K48, ubiquitin chains activate RIG-I(N). Ubiquitin chains of defined lengths and linkages were incubated with GST-RIG-I(N), then IRF3 dimerization assay was carried out as in (A). The quality of the ubiquitin chains was evaluated by immunoblotting (lower panel) or silver staining (see Figure S4). (C) K63 ubiquitin chains potently activate RIG-I(N) in a chain length- and linkage-dependent manner. Different concentrations of Ub chains (0.01–1 μM) or ubiquitin (0.1–10 μM) were tested for RIG-I(N) activation using the IRF3 dimerization assay. (D) Similar to (C), except that full-length His6-RIG-I and 5′-pppRNA were used in lieu of RIG-I(N) in the reactions. (E & F) IRF3 dimer in C & D, respectively, was quantified using ImageQuant, then plotted against the concentration of Ub or Ub chains.