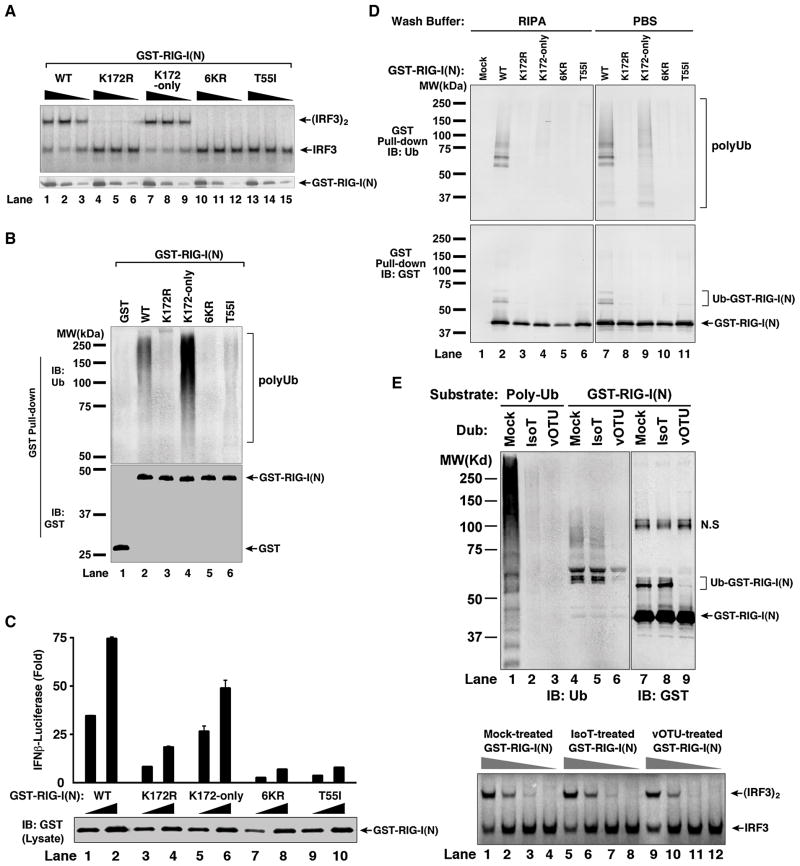
Figure 6. Polyubiquitin Binding is Required for RIG-I Activation.
(A) Mutations at K172 and T55 impair RIG-I(N) activation in vitro. GST-RIG-I(N) and the indicated mutants were expressed and purified from E. coli, then incubated with K63-Ub4, followed by IRF3 dimerization assay (upper panel). Aliquots of the reaction mixtures were analyzed by immunoblotting with a GST antibody (lower panel). 6KR: GST-RIG-I(N) containing six K>R mutations, including K172R. K172-only: similar to 6KR except that K172 is not mutated. (B) Mutations at K172 and T55 impair polyUb binding by RIG-I(N) in vitro. GST-RIG-I(N) and mutant proteins were incubated with K63 polyUb chains, then pulled down with glutathione-Sepharose and analyzed by immunoblotting. (C) Mutations at K172 and T55 impair RIG-I(N)’s ability to induce IFN-β in cells. Different amounts (30 ng and 100 ng) of mammalian expression vectors for GST-RIG-I(N) and mutants were transfected into HEK293-IFNβ-Luc cells, then luciferase activity was measured. Error bars represent the variation range of duplicate experiments. (D) GST-RIG-I(N) and mutant proteins as described in (C) were pulled down with glutathione-Sepharose beads, washed with PBS or RIPA buffer, then analyzed by immunoblotting. (E) GST-RIG-I(N) was expressed in HEK293T cells, then purified using glutathione-Sepharose. The purified protein was treated with isopeptidase T (IsoT) or viral OTU (vOTU) or mock-treated, then immunoblotted with an antibody against ubiquitin or GST (upper panel; N.S indicates a non-specific band). Aliquots of the treated GST-RIG-I(N) were tested for its ability to promote IRF3 dimerization in the reconstitution assay (lower panel). See also Figure S6.