Abstract
Background
Understanding the mechanisms of repair and regeneration of the kidney following injury is of great interest because there are currently no therapies that promote repair, and kidneys frequently do not repair adequately. We studied the capacity of human CD34+ hematopoietic stem/progenitor cells (HSPCs) to promote kidney repair and regeneration using an established ischemia reperfusion injury model in mice, with particular focus on the microvasculature.
Methods and Results
Human HSPCs administered systemically 24h following kidney injury were selectively recruited to injured kidneys of immunodeficient mice and localized prominently in and around vasculature. This recruitment was associated with enhanced repair of the kidney microvasculature, tubule epithelial cells, enhanced functional recovery and increased survival. HSPCs recruited to kidney expressed markers consistent with circulating endothelial progenitors, and synthesized high-levels of pro-angiogenic cytokines, which promoted proliferation of both endothelial and epithelial cells. Although purified HSPCs acquired endothelial progenitor markers once recruited to the kidney, engraftment of human endothelial cells in the mouse capillary walls was an extremely rare event, indicating HSC-mediated renal repair is by paracrine mechanisms rather than replacement of vasculature.
Conclusions
These studies advance human HSPCs as a promising therapeutic strategy for promoting renal repair following injury.
Keywords: human hematopoietic stem cells, kidney, repair, regeneration, angiogenesis
Introduction
Although the kidney has tremendous capacity for regeneration, chronic kidney disease and kidney failure following acute kidney injury or following both repetitive and chronic kidney injuries are now leading causes of morbidity and mortality in the world 1–3. Furthermore chronic kidney disease has been identified as a leading independent risk factor for cardiovascular diseases and cardiovascular mortality 4. Chronic kidney diseases may represent unsuccessful or inadequate renal repair following injury, and currently there are few therapies that promote repair and regeneration of the kidney 5.
There has been much interest in the reparative and angiogenic properties of stem cells from bone marrow 6–8, and several studies in mouse models of kidney disease have shown that mouse mesenchymal stromal cells of bone marrow (MSCs) can prevent or attenuate kidney injury, possibly by paracrine or systemic secretory mechanisms 9–11. However the possible angiogenic role of hematopoietic stem/progenitor cells (HSPCs) in kidney repair has been little explored and no studies have ascertained the practicability of harvesting human HSPCs in cell therapy to promote organ repair and regeneration 12.
The kidney peritubular microvasculature has received increasing attention recently, since this fragile vasculature may not regenerate normally following injury. This may predispose to chronic ischemia of the kidney 13–16 triggering chronic inflammation, tubular atrophy, and interstitial fibrosis, hallmarks of chronic kidney disease 13–15. It has been proposed that successful regeneration of peritubular capillaries following injury is central to progression to chronic kidney diseases 13–15.
The fact that human stem cells from bone marrow may have angiogenic properties, have the capacity to differentiate into a primitive cell type, known as circulating endothelial progenitor (CEP) that is recruited to sites of blood vessel injury to help repair damage 17–20, and have been shown to promote vascular regeneration in other organs 21–23 led us to study the role of human HSPCs in kidney repair following injury, with particular attention to the peritubular capillary plexus.
In these studies we show that mobilized human CD34+ stem/progenitor cells are recruited to the injured kidney and promote survival, vascular regeneration and functional recovery.
Methods
An expanded version of Materials and Methods section is available in online data supplement and includes detailed methods for the following: Animals; Human Peripheral Blood CD34+ Cell Purification and Tracking; Animal Model; Renal Function; Tissue Preparation, Immunostaining, Imaging and Quantification of Injury and Repair; Flow Cytometric Analysis and Sorting; Real Time PCR.
Statistical Analysis
All values are given as mean ± standard deviation (SD). Mantel-Cox Log-rank test was used to analyze survival. The nonparametric Mann-Whitney U test was used for group comparisons. Analyses were performed using Prism software (Graphpad). For antibody cytokine arrays results were normalized to positive controls, background signal was removed by subtracting the average of the negative controls plus two standard deviations. P values less than 0.05 were considered significant in all statistical tests.
Results
Characterization of Isolex-purified G-CSF-mobilized hematopoietic stem/progenitor cells
CD34+ enriched leukocytes from HSC-mobilized human donors were analyzed for viability and purity. More than 98% of HSPCs were viable when assessed by exclusion 7-amino actinomycin-D (Figure S1A). More than 96% of leukocytes were CD45+, CD34+ indicating they were HSPCs (see the online-only Data Supplement Table 1). Further, they exhibited the capacity to differentiate into all hematopoietic lineages confirming the HSPCs to contain stem cells as well as progenitor cells (Figure S2). A minority expressed CD34 but lacked CD45. Further characterization of the enriched leukocytes was performed using the cell surface markers CD14, CD34, CD146, CD133, CD31, VEGFR2 for confirmation of multi-lineage potential and identification of putative endothelial progenitors (see the online-only Data Supplement Table 2) 20. The characterization, in addition to HSPCs, is consistent with mobilized human peripheral blood CD34+ cells containing small numbers of CEPs and possibly rare circulating endothelial cells (CECs) 20, 24, 25.
Human hematopoietic stem/progenitor cells are recruited to kidney during repair following ischemia reperfusion injury
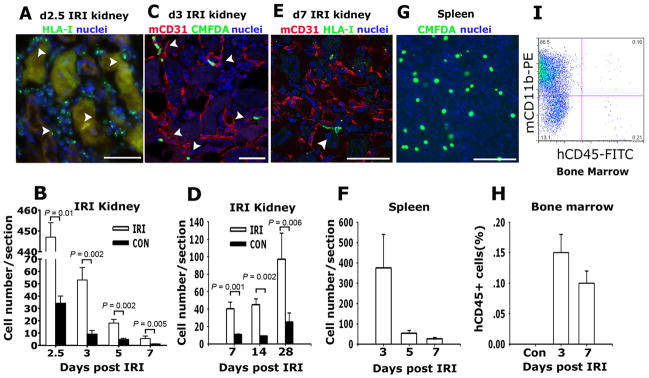
To study the effect of human HSPCs on kidney repair we initially determined whether they could be recruited to the injury kidney. In preliminary studies I.V. infusion of 2.5 ×106 HSPCs labeled with CMFDA prior to injury did not result in significant recruitment 24h after injection (data not shown). Next we infused CMFDA-labeled HSPCs on day 1 and 2 after kidney IRI, and looked in the kidney 2.5, 3, 5, 7 days following injury (Figure 1) where many recruited HSPCs could be detected in the kidney parenchyma. Many were localized within peritubular capillaries (PTC) (Figure 1C), but some were detected outside of the confines of the capillaries in a perivascular location (Figure 1E and Figure S1B). We also noticed that following unilateral IRI there was a small but significant recruitment of HSPCs to the uninjured kidney (Figure 1B). However we could not detect any HSPCs in the heart or gut (not shown) indicating that this was specific recruitment of HSPCs to the uninjured and injured kidney. Due to concern that CMFDA might be diluted and become undetectable with time we infused unlabeled HSPCs into mice on d1 and d2 following injury. These unlabeled cells were detected by antibodies against HLA class I (Figure 1A,D,E). HLA-I positive cells were readily detected in the kidneys at all time points but notably there was persistence of HLA-I+ cells in the kidney 14 and 28 after IRI (Figure 1D). As expected, HSPCs were also identified in spleen and bone marrow (Figure 1F–H), and there was persistence of HSPCs in the marrow, with evidence on d7 following IRI that HSPCs in the bone marrow had induced the myeloid marker CD11b (Figure 1I) suggesting that HSPCs had engrafted the mouse bone marrow and that the mice were now chimeric.
Figure 1.
Human hematopoietic stem cells are recruited to post ischemia reperfusion injury kidneys, spleen and bone marrow of NOD/SCID mice. (A) Photomicrograph of NOD/SCID mouse d2.5 post IRI kidney that received adoptively transferred human HSPCs on d1, showing HLA-I positive cells in the interstitium of the post IRI kidney (arrowheads) between necrotic tubules. (B) Graph indicating the number of human CMFDA+ HSPCs identified in post IRI and control kidneys with time after IRI. (C) Representative confocal image of day 3 post IRI kidney following IV transferred of human HSPCs on d1 post IRI showing CMFDA fluorescing human cells (arrowheads) in peritubular CD31+ capillaries. (D) Graph indicating the number of human HLA class I cells in post IRI and control kidneys on days 7, 14 and 28 after IRI. (E) Representative confocal image detecting human HLA class I (green, arrow) of day 7 post IRI kidney of NOD/SCID mice treated with human HSPCs d1 after IRI. (F) Representative confocal image of CMFDA labeled human HSPCs in spleen 3d following IRI, adoptively transferred 1d after kidney IRI. (G) Graph indicating the number of CMFDA positive cells per section in the spleen on days 3, 5 and 7 after IRI. (H) Graph indicating proportion of human CD45+ cells in mouse bone marrow following adoptive transfer. (I) Representative flow cytometric plot for CD11b (detects mouse and human antigens) and human CD45 of whole bone marrow from d7 post kidney IRI mouse that received adoptively transferred human HSPCs d1 after IRI. Mean ± SD. **P < 0.01 vs. control kidney. (Bars, 50μm, n = 6–10/group)
Systemic human hematopoietic stem/progenitor cell therapy reduces mortality and improves kidney function following ischemia reperfusion injury
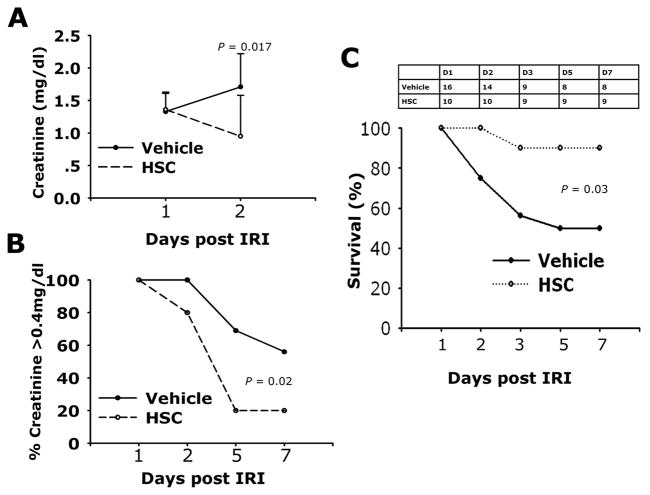
To determine whether HSC recruitment to the injured kidney had any functional consequence during repair, we subjected mice to bilateral IRI (day 0), followed by intravenous infusion of human HSPCs on d1 and d2. Plasma creatinine was assessed in sham surgery mice (d0, plasma creatinine value is 0.05 ± 0.06) and on d1, d2, d5, and d7 following IRI. Bilateral kidney IRI resulted in significant increase in serum creatinine at 24 hours and peaked at 48h (Figure 2A). Although plasma creatinine levels at 24 hours (time of first injection) were no different in treatment and vehicle groups, there was a marked and significant decrease in plasma creatinine at 48h in mice that had received HSPCs (Figure 2A), while the vehicle group of mice had persistently highly elevated plasma creatinine levels at this time. Over the subsequent five days of recovery, the proportion of mice that showed recovery of kidney function detected by a plasma Cr ≤ 0.4mg/dL was significantly higher in mice treated with HSPCs (Figure 2B). In the vehicle treated group, only 50% of mice survived to day 7, whereas 90% of mice that received human HSPCs survived to day 7 (Figure 2C). The surviving numbers in the two groups can be seen in Figure 2C. These findings indicate that human HSPCs both promote kidney repair/regeneration and enhance survival.
Figure 2.
Adoptive transfer of human HSPCs to NOD/SCID mice following kidney ischemia reperfusion injury decreases mortality and improves kidney function. (A) Plasma creatinine levels on days 1 and 2 following bilateral IRI followed IV injection with PBS (Vehicle, n=16) or 2.5×106 (HSC, n=10) 1day following injury. Data are mean ± SD. (B) Curves showing the proportion of mice with plasma creatinine ≤0.4 mg/dL at each time point following IRI. (C) Survival curves and number at each time point, for mice undergoing bilateral IRI followed IV injection with PBS (vehicle) or 2.5×106 human HSPCs (HSC) 1 day following injury.
Human hematopoietic stem/progenitor cell therapy attenuates kidney peritubular capillary loss, promotes tubular epithelial regeneration and prevents long-term fibrosis following ischemia reperfusion injury
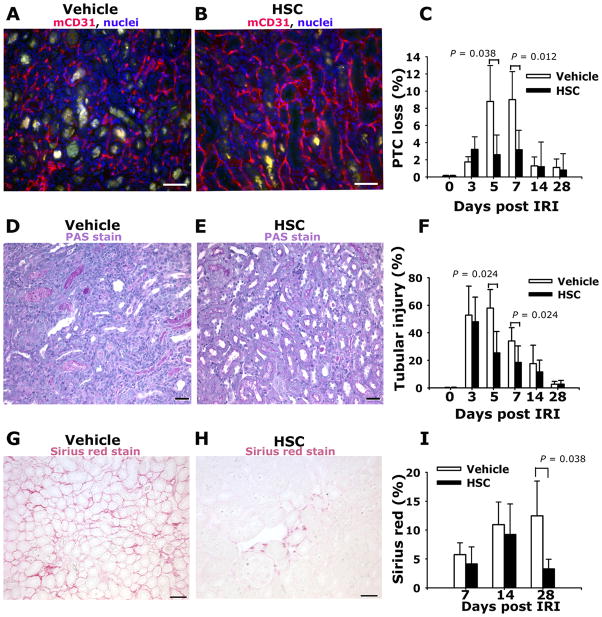
To study the mechanism by which HSPCs promote kidney repair we analyzed kidney sections for loss of peritubular capillaries (PTCs) and persistence of tubule injury (Figure 3). Analysis of mCD31-labeled PTCs by morphometry revealed that HSC treatment prevented PTC loss (Figure 3A–C) during the repair phase through d7 post IRI. PTC loss after 14 and 28 days was not different in HSC treated mice indicating that there are endogenous factors promoting regeneration of PTCs, but that HSC therapy attenuates early loss of vasculature. Similarly, HSC therapy attenuated persistence of tubule injury during the repair phase of this model of IRI (Figure 3D–F), suggesting that HSPCs promoting tubule regeneration by either direct or indirect mechanisms. We have previously demonstrated that kidney IRI can lead to persistent interstitial fibrosis, a harbinger of chronic kidney disease and strongly associated with progressive long-term loss of kidney function 15, 26–28. To test whether systemic infusion of HSPCs during repair of the injured kidney affected long-term consequences of injury we quantified interstitial fibrosis (Figure 3G–I). In vehicle treated mice, interstitial fibrosis progressively accumulated in the four weeks following injury but in those mice that had received HSPCs interstitial fibrosis was attenuated by d28.
Figure 3.
Adoptive transfer of Human HSPCs attenuates peritubular capillary loss and reduces tubular epithelial injury following kidney ischemia reperfusion injury. (A–B) Representative images of mouse CD31-labeled peritubular capillaries (PTC) of outer medulla of d7 post IRI kidney that received vehicle (A) or HSPCs (B) on d1 and d2. Note marked PTC loss in (A). (C) Graph showing PTC index for mice following vehicle or HSPCs (n=3 per timepoint). (D–E) Representative light images of PAS stained kidney sections of outer medulla d5 post IRI kidney from mice that received vehicle (D) or HSPCs (E) on d1 and d2. Note prominent debris in severely injured tubules in (D), present to a much lower extent in (E). (F) Graph showing tubular injury index for mice following vehicle or HSPCs (n = 6–10 per timepoint). (G–H) Representative images of Sirius red-stained kidneys d28 post IRI that received either vehicle (G) or HSPCs (H) on d1 and d2 post IRI. (I) Graph showing fibrosis area for mice following vehicle or HSPCs (n = 6–10 per timepoint). Mean ± SD. *P < 0.05, vs. vehicle group. (Bars = 50μm).
Human hematopoietic stem/progenitor cells acquire endothelial progenitor cell markers in the kidney following ischemia reperfusion injury
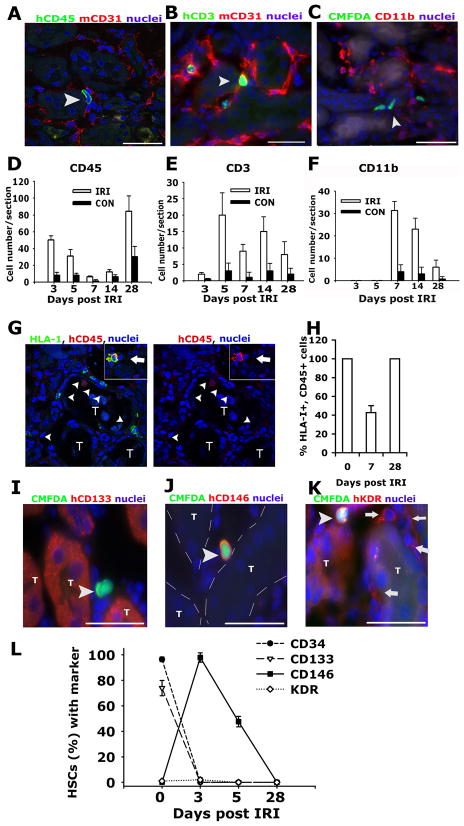
HSPCs are the source of myeloid, erythroid, megakaryocyte and lymphoid lineage cells. We noted that while many HSPCs were recruited to kidneys on d2 and d3 after injury, the number of retained cells fell progressively through d7, but thereafter increased up to d28 after injury (Figure 1). We labeled kidneys for human lymphoid and myeloid commitment markers (Figure 4). CD3 was expressed in a minority of HSPCs recruited to the kidney (Figure 4B, E) and this expression occurred several days following recruitment and was proportionately similar in both uninjured and injured kidney. None of the recruited HSPCs expressed the myeloid differentiation marker CD11b in the first 5d following injury (Figure 4C, F). Most HSPCs recruited to the kidney during the week following injury do not therefore differentiate into mature leukocyte lineages. The number of human cells in the kidney increases late after injury, that is after d7. From d14, CD11b was expressed in human cells in the kidney indicating that mature human myeloid cells were present in the kidney. Furthermore, on d28 human cells uniformly express the leukocyte common antigen, CD45 (Figure 4H) but lack CD34 (Figure 4L), and occasional human cells with characteristic multilobar nuclei of neutrophils were identified in d14 and d28 post IRI kidneys (not shown). Collectively, these findings indicate that the late increase in human cells in the kidney either reflects bone marrow chimerism and recruitment of mature cells from bone marrow, or reflects local differentiation of mature cell types in the kidney.
Figure 4.
Differentiation of human HSPCs in kidneys. Representative confocal image (A) and epifuorescence images (B–C) of kidney outer medulla showing expression of human CD45 (A), human CD3 (B), and absence of CD11b (C) in cells (arrowheads) in d5 post IRI kidneys that received IV injection of HSPCs 1 day following injury. Images are co-labeled (A–B) to show mCD31 (red) of the mouse vasculature. Graphs showing the number of human CD45+ (D), human CD3+ (E), and CD11b+; CMFDA+ (F) cells identified in post IRI kidneys and control kidneys. (G–H) Confocal split panel image (G) and graph (H) showing the presence of HLA-I+, hCD45− cells (arrowheads) in the interstitium of the d7 post IRI kidney [T = tubule]. Inset shows a HLA-I+, hCD45+ leukocyte (arrow). (I–K) Representative epifluorescence images of the outer medulla of day 3 post IRI kidney of NOD/SCID mice that received human HSPCs on d1–d2 labeled with CMFDA (arrowheads) co-labeled with antibodies against human CD133 (I), CD146 (J) and KDR (K). Note anti-KDR antibodies also detected mouse endothelium (arrows) [T = tubule, outlined with hatched white line in J]. (L) Graph showing the expression of stem cell/CEP markers by HSPCs prior to (d0) and following recruitment to the post IRI kidney. (Bars = 50μm). Data are mean ± SD. n = 6/timepoint.
To investigate further the local differentiation of HSPCs in the kidney during the first 7d, sections were initially co-labeled to detect human CD45 with HLA-I (Figure 4G–H). Although d0 HSPCs uniformly expressed both markers, a proportion of HSPCs recruited to the kidney did not express CD45, 7d post IRI. Kidneys were next labeled to detect human CD34, and CEP markers VEGFR2 (KDR), CD146 and CD133, and this expression was compared with d0 HSPCs (Figure 4I–L). While few mobilized enriched HSPCs expressed KDR or CD146, the majority expressed CD133 prior to injection. In the kidney, however, d3 post IRI there was a phenotypic switch as nearly all recruited HSPCs expressed CD146, but none expressed CD133 (Figure 4L). The expression of KDR was similar in mobilized, enriched HSPCs compared with those recruited to kidney. Since CD146 expression has been associated with CEP functions 29 and since few HSPCs express the T cell receptor (Figure 4E), our findings suggest that the kidney promotes HSC differentiation toward CEP type function. Since a small fraction of the purified HSPCs expressed CD146 prior to injection into mice we tested whether the apparent phenotypic switch was due to selective recruitment of CD146+, CD34+ cells to the kidney after infusion. Mobilized purified HSPCs were separated into CD146+ fractions and CD146− fractions. Aliquots of these subpopulations were infused intravenously into mice 24h after kidney IRI (see the online-only Data Supplement Table 2). There was equivalent recruitment of the CD146+ or CD146− cells to the injured kidney. Furthermore, the CD146− cells rapidly acquired expression of CD146, confirming that HSPCs rapidly undergo a phenotypic switch in kidney.
Human hematopoietic stem/progenitor cells contribute to vascular repair by paracrine mechanisms
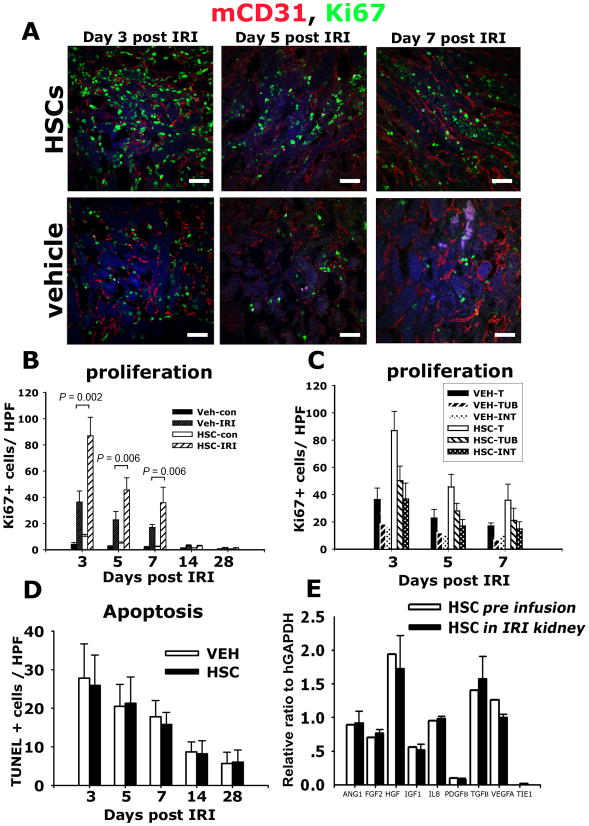
To dissect the mechanism by which HSPCs support neovascularization, we determined initially whether HSPCs had differentiated into endothelial cells. Using the human-specific antibodies against CD31 and human vWF, two markers of endothelial cells, we identified human CD31+ cells in injured kidneys at day 7, 14 and 28, but not at earlier timepoints (Figure 5A, B, C). Therefore CD31 expression did not coincide with maximal repair. Occasional CD31+ HSPCs lacked CD45 expression and were found in the PTC wall with morphology consistent with endothelial cells (Figure 5A). However the vast majority of CD31+ human cells also co-expressed CD45 (Figure 5B) or were located in the interstitium with leukocyte morphology, consistent with CD31 expression by lymphocytes and monocytes, and indicating that human CD31 is not a specific marker of endothelium. Parallel studies using anti-human vWF antibodies (that did not cross react with mouse vWF) also identified very rare vWF+ human cells which lacked CD45 expression (Figure 5D), adding weight to the observation that occasional human cells do become functioning endothelial cells. Since these investigations provided evidence for only extremely rare contribution of purified human CD34+ cells to direct endothelial replacement, yet there was marked expression of the CEP marker CD146 in all HSPCs (Figure 4), we tested whether HSPCs were functioning by paracrine mechanisms. This was particularly tractable given the intra and perivascular locale of HSPCs in the kidney following injury. Kidneys labeled for cells in cell-cycle using the pan cell cycle marker Ki67 was revealing: infusion of HSPCs led to a marked increase in the number of parenchymal cells in cell-cycle following injury (Figure 6A-C), and this enhancement of cellular proliferation persisted throughout the repair phase. The enhanced proliferation was both in tubule epithelial cells and interstitial cells indicating that HSPCs were influencing both compartments (Figure 6C). In addition, in the unilateral model of IRI where there is compensatory growth in the uninjured kidney the HSPCs also promoted proliferation of parenchymal cells of this kidney. While proliferation was enhanced there was no change in the number of TUNEL+ apoptotic cells seen in the kidneys (Figure 6D). To explore further potential release of cytokines locally, CMFDA-labeled HSPCs that had been recruited to the kidney on d4 post IRI were purified from whole kidney using established methods 30 and their human specific transcriptional profile was analyzed by RT-PCR comparing it to the transcriptional profile of homogeneic HSPCs prior to systemic injection into mice (Figure 6E). Mobilized, enriched HSPCs generated high levels of transcripts for pro-angiogenic cytokines including ANG-1, FGF-2, and VEGF-A, and in addition generated high levels of HGF recognized for its role in kidney epithelial regeneration (Figure 7). Strikingly, after injection into mice, those HSPCs that were recruited to the kidney exhibited highly similar transcriptional activity for the pro-angiogenic cytokines, further supporting a paracrine role in angiogenesis.
Figure 5.
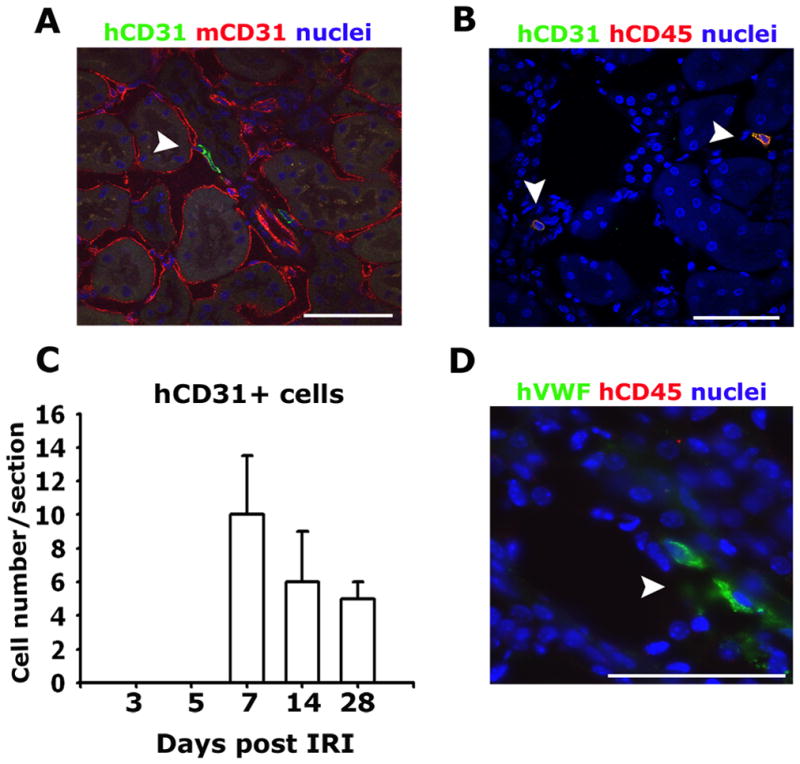
Rare human endothelial cells are detected in the kidney after ischemic injury and HSC infusion. (A–B) Confocal images of d28 post IRI kidneys showing the presence of human CD31 expressing cells some of which appear to be integrated into capillaries (arrowhead) (A), but the majority are morphologically monocytic and co-express hCD45 (arrowheads) (B). (C) Graph showing the number of human CD31 expressing cells in the post IRI kidneys with time following adoptive transfer of HSPCs 1day following injury. (D) Specific expression of human von Willebrand factor (vWF) (arrowheads) and not mouse vWF in cells that lack expression of human CD45 in the post IRI kidney (Bar = 50μm).
Figure 6.
Human HSPCs generate angiogenic paracrine factors in the kidney after ischemia resperfusion injury that promote parenchymal cell proliferation. (A–C) Confocal images (A) and quantification (B–C) of Ki67+ cells in kidney (B), or kidney tubule (TUB) or interstitial (INT) compartments (C). (D) T.U.N.E.L. positive apoptotic cells in kidney sections post IRI. (E) Relative gene expression compared with GAPDH of pro-angiogenic transcripts in mobilized HSPCs prior to transfer to mice (white) and those purified from post-IRI kidney 48h following transfer to mice. Note that HSPCs recruited to the kidney retain high-level expression of pro-angiogenic transcripts. Mean ± SD.**P < 0.01 n = 6/group. (Bars = 50μm).
Figure 7.
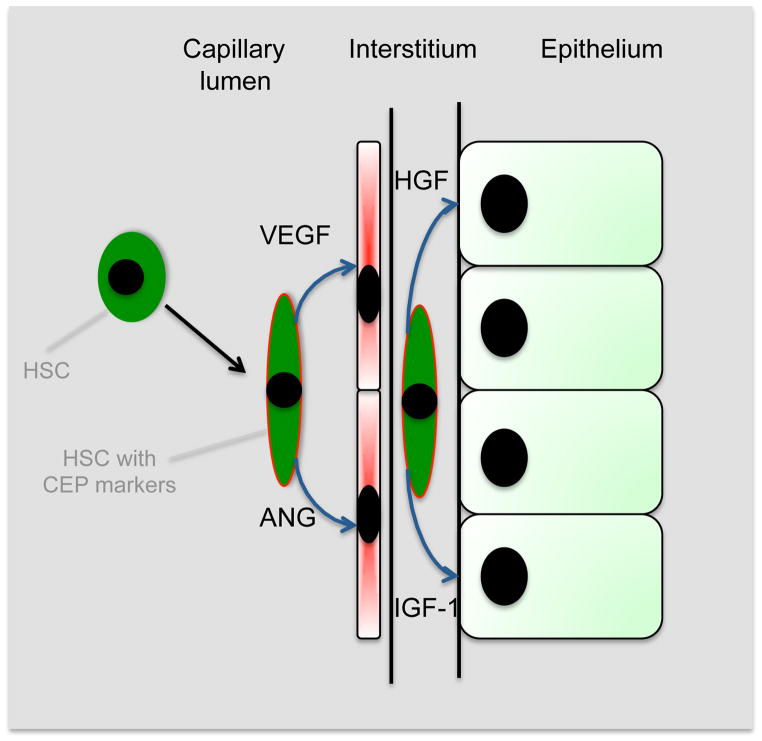
Model of functions of HSPCs in repair of the kidney following injury. HSPCs are recruited to the injured kidney where they acquire the CEP marker CD146 and localize within injured capillaries and in the interstitium. Local production of cytokines including Angiopoietins, Vascular endothelial growth factors, hepatocyte growth factor and insulin like growth factors are generated promoting cellular repair by paracrine mechanisms.
Since HSPCs were also recruited to spleen (Figure 1) we determined whether HSPCs secreted circulating angiogenic cytokines in addition to local production of angiogenic factors directly in the kidney. Protein microarray analysis of plasma detected angiogenic cytokines at enhanced levels in mice that had received HSPCs (Figure S3A) and these were context specific, that is plasma levels of human angiogenic cytokines were affected by the presence of IRI disease in the kidney. However the levels were low, compared with endogenous production of the same cytokines. In addition, cultured spleen cells did not generate angiogenic cytokines (Figure S3B), making the spleen an unlikely source of angiogenic factors, and suggesting that the kidney may be the major source of circulating human angiogenic factors. Since human angiogenic factors were nevertheless detected in the circulation we infused conditioned media from 2.5 ×106 cultured HSPCs into mice d1 post IRI to determine whether HSPCs function as endocrine cells. Whereas HSC infusion promoted a rapid decline in creatinine level (Figure 2), conditioned media resulted in a non-significant improvement of renal function (Figure S3C), indicating that detection of human angiogenic cytokines in plasma likely reflects the paracrine rather than endocrine action of HSPCs in the kidney.
Discussion
Acute kidney injury in humans continues to confer high mortality and has limited therapeutic options, therefore identifying potential regenerative approaches, as new therapeutic strategies, is highly desirable. In addition, emerging evidence indicates that acute kidney injury in humans is a harbinger of chronic kidney disease characterized by inflammation, vasculopathy, epithelial atrophy, fibrosis and progressive loss of function leading to organ failure 3, 15, 27, 31. New strategies that attenuate kidney injury or enhance repair and regeneration will not only have short-term impact but conceivably will alter the long-term course for kidney function. The long-term benefits from for such therapies will impact not only kidney disease but also cardiovascular diseases, since chronic kidney disease is an independent risk factor for cardiovascular diseases 4. Recently, adult human peripheral blood CD34+ cells as well as HSPCs have been reported to promote vasculogenesis and osteogenesis following stroke and bone injury 22, 32. Furthermore, CD34+ cells are capable of expansion and mobilization into the peripheral circulation in the presence of exogenously applied G-CSF 33–35, making HSPCs readily available, and strengthening the rationale of clinical cellular therapy.
In the present study, we demonstrated that human HSPCs administered systemically 24h following kidney injury were selectively recruited to injured kidneys and localized prominently within and around vasculature. This recruitment was associated with enhanced repair of the microvasculature, tubule epithelial cells, enhanced functional recovery, and increased survival of mice. Additionally, long-term fibrosis was prevented.
During the first 7d of regeneration and recovery post IRI, most HSPCs recruited to the kidney did not acquire markers of myeloid or lymphoid differentiation, rather they acquired markers consistent with CEPs 20, 36. Further, HSPCs synthesized high-levels of pro-angiogenic transcripts and this pattern of transcription persisted following recruitment to the kidney, which is another characteristic of CEPs. Although mobilized-purified HSPCs contained small numbers of CEPs and rare CECs defined by cell surface markers prior to recruitment to the kidney, the injured kidney environment triggered HSPCs to lose CD133, CD45 and activate expression of CD146, phenotypic features consistent with CEP differentiation at the site of injury. This injury-triggered differentiation toward CEP cell-type is highlighted by the fact that HSPCs recruited to spleen did not generate angiogenic cytokines. Nevertheless, despite local CEP differentiation within the kidney we identified very few human endothelial cells in the mouse capillary walls. Taken together these data indicate that HSC-mediated renal repair is by paracrine mechanisms rather than replacement of vasculature (Figure 7). The definition of CEPs or even their existence remains controversial 19, 37. The data presented here show, at least in kidney injury, that human HSPCs can remain in a peripheral organ as a primitive cell type for several days and promote vascular repair, and that this cell-type loses CD34 and expresses cell surface markers that others have reported in cells performing vascular reparative functions 17. Our findings are consistent therefore with HSPCs becoming CEPs, but not becoming angioblasts and replacing lost endothelial cells directly. Our studies very rarely identified cells within the capillary wall with endothelial morphology, expression of CD31, vWF and absence of CD45 38, consistent with human endothelial cells. CD31 expression alone is insufficient to designate a cell endothelial since it is also expressed by B cells and monocyte/macrophages 39. Mobilized purified HSPCs contained a minor population of purified CECs (<0.05%) 40, 41. It is possible therefore that the rare human endothelial cells detected in mouse kidney derived from cell fusion of CEPs with endothelial cells, incorporation of rare CECs, or possibly occasional transdifferentiation of CEPs.
Human HSPCs were selectively recruited into injured kidney indicating that the kidney releases chemoattractants for HSPCs 12, 42, 43. Systemic administration of HSPCs at the onset of injury (d0) led to poor recruitment of HSPCs, but delayed administration of HSPCs until the beginning of the repair phase was highly effective in triggering recruitment. This recruitment pattern is similar to monocyte influx to the kidney, suggesting that in addition to SDF-1, other monocyte chemoattractants may play a role in HSC recruitment. Our prior studies in mice provided no evidence for endogenous HSC mobilization from the bone marrow or recruitment to the kidney, simply in response to IRI, although others have reported that endogenous HSC can be recruited to injured kidney 10, and the differences in these data may be methodological. Regardless of whether endogenous HSPCs traffic to the kidney following injury, our studies indicate that there is an inadequate endogenous signal for recruitment of HSPCs from their normal niche in the bone marrow 44. Since injection of HSPCs into the peripheral circulation results in effective recruitment to the kidney, HSC therapy overcomes a normal block in release from the bone marrow niche.
HSPCs were detected in the kidney through d14 and d28 after IRI, using antibodies against HLA-class-I antigens. There was a bimodal distribution of HSC retention in the kidney with time, with the nadir occurring at about seven days. We noted that the mice developed bone marrow chimerism, and that at d14 and d28 (but not earlier) all of the human cells in the kidney expressed mature lineage markers, and none of them expressed stem cell markers. It is likely therefore that many of the late human cells in the kidney are recruited from bone marrow rather than deriving from the original recruitment of HSPCs. However, our data strikingly point to HSC infusion on d1 and d2 of disease resulting in long-term impact on fibrosis. It is unclear from these current studies whether a reduction in long-term fibrosis reflects improved early vascular repair or whether it reflects a persistent population of reparative mature human leukocytes in the kidney at late time-points.
Ischemic injury in the kidneys is characterized by epithelial injury. Less well described is the loss of PTC. But, data derived from several severe acute kidney injury models (ischemia, toxin, transient angiotensin II) demonstrate capillary loss that typically precedes the development of prominent fibrosis 15, 16, 45, and neoangiogenesis may be a central process in preservation of vascular structure and restoration of organ function 13, 14, 46, 47. We also show in these studies that following IRI there is marked loss of PTCs with only relatively mild renal injury and that although there is significant regeneration of these PTCs during repair, there is persistent loss of vasculature one month after injury, indicating that the kidney has an inherent defect in revascularization after injury 15, unlike other organs such as skin. Our studies show unequivocally that HSPCs attenuate that loss of PTCs in the kidney during the repair, and this is associated with both rapid functional recovery of the kidney and enhanced survival of mice. In our unilateral IRI model, HSC-mediated regeneration of PTCs did not attenuate the long-term persistent PTC loss at 28 weeks, but nevertheless impacted on recovery and survival seen in the bilateral IRI model, pointing to early vascular repair as a central process in renal repair. Despite the window of HSC-mediated vascular repair being restricted to early time-points after injury, there was nevertheless prevention of fibrosis progression in the kidney at one month after injury. Further studies will be required to understand whether this long-term effect of early HSC infusion is through early enhancement of pericyte-endothelial cell interactions which may be a central interaction in the development of interstitial fibrosis 48, 49. In preliminary studies late administration of HSPCs to mice 14 days post IRI kidney resulted in poor recruitment and little evidence of enhanced vascular repair (not shown), indicating that there is a restricted period post injury during which HSPCs are efficacious.
Although the kidney IRI model is characterized by severe injury and repair of the tubule epithelial cells, particularly the S3 segment of the proximal tubule, it is likely that without PTC regeneration those injured tubules will not regenerate successfully due to persistent ischemia 15, 50. In addition to PTC regeneration, HSPCs promoted epithelial regeneration, as assessed by tubule injury score and functional recovery. The generation of angiogenic cytokines, combined with the intravascular and perivascular locale of HSPCs suggests a primary role in kidney microvascular repair, which secondarily promotes epithelial repair. However since HSPCs generate HGF which is known to promote epithelial regeneration directly, HSPCs may have direct paracrine role on epithelial repair, independently of PTC repair.
In conclusion, we demonstrate here that systematically administered peripheral blood mobilized human HSPCs reduce mortality and promote rapid renal repair and regeneration of the kidney by paracrine mechanisms directed at peritubular capillaries. These findings support human HSPCs as a promising therapeutic strategy for treatment of acute kidney diseases, and in the prevention of chronic kidney diseases.
Supplementary Material
Acknowledgments
Thanks to Brian Nowlin (HMS), Dr Huaying Pei (HMS), Dr. Ana Castano (HMS) for assistance. Thanks to Dr. Cathy Hoff (Baxter) for advice and review of manuscript and Prof. Baofeng Yang (Harbin Medical University) for continued support.
Sources of Funding
The Duffield laboratory is funded by NIH grants DK73299, DK84077, and DK87389, and a Sponsored Research Agreement from Baxter. Dr. Li was additionally supported by the National Natural Science Foundation of China No.30771006, Heilongjiang Overseas Scholar Foundation 2006, China Postdoctoral Foundation 2007 and by the Scientific Research Foundation of State Education Ministry for the Returned Overseas Chinese Scholars.
Footnotes
Disclosures
Baxter has a patent pending for the use of human stem cells in repair of the injured kidney. Dr. Duffield serves on the Scientific Advisory Board for Promedior Inc.
References
- 1.Ali T, Khan I, Simpson W, Prescott G, Townend J, Smith W, Macleod A. Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J Am Soc Nephrol. 2007;18:1292–1298. doi: 10.1681/ASN.2006070756. [DOI] [PubMed] [Google Scholar]
- 2.Meguid El Nahas A, Bello AK. Chronic kidney disease: the global challenge. Lancet. 2005;365:331–340. doi: 10.1016/S0140-6736(05)17789-7. [DOI] [PubMed] [Google Scholar]
- 3.Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med. 1996;334:1448–1460. doi: 10.1056/NEJM199605303342207. [DOI] [PubMed] [Google Scholar]
- 4.Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL, Salem DN, Levey AS, Sarnak MJ. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol. 2004;15:1307–1315. doi: 10.1097/01.asn.0000123691.46138.e2. [DOI] [PubMed] [Google Scholar]
- 5.Sagrinati C, Ronconi E, Lazzeri E, Lasagni L, Romagnani P. Stem-cell approaches for kidney repair: choosing the right cells. Trends Mol Med. 2008;14:277–285. doi: 10.1016/j.molmed.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Li B, Morioka T, Uchiyama M, Oite T. Bone marrow cell infusion ameliorates progressive glomerulosclerosis in an experimental rat model. Kidney Int. 2006;69:323–330. doi: 10.1038/sj.ki.5000083. [DOI] [PubMed] [Google Scholar]
- 7.Togel F, Cohen A, Zhang P, Yang Y, Hu Z, Westenfelder C. Autologous and allogeneic marrow stromal cells are safe and effective for the treatment of acute kidney injury. Stem Cells Dev. 2009;18:475–485. doi: 10.1089/scd.2008.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Huyen JP, Smadja DM, Bruneval P, Gaussem P, Dal-Cortivo L, Julia P, Fiessinger JN, Cavazzana-Calvo M, Aiach M, Emmerich J. Bone marrow-derived mononuclear cell therapy induces distal angiogenesis after local injection in critical leg ischemia. Mod Pathol. 2008;21:837–846. doi: 10.1038/modpathol.2008.48. [DOI] [PubMed] [Google Scholar]
- 9.Togel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol. 2007;292:F1626–1635. doi: 10.1152/ajprenal.00339.2006. [DOI] [PubMed] [Google Scholar]
- 10.Bi B, Schmitt R, Israilova M, Nishio H, Cantley LG. Stromal cells protect against acute tubular injury via an endocrine effect. J Am Soc Nephrol. 2007;18:2486–2496. doi: 10.1681/ASN.2007020140. [DOI] [PubMed] [Google Scholar]
- 11.Imberti B, Morigi M, Tomasoni S, Rota C, Corna D, Longaretti L, Rottoli D, Valsecchi F, Benigni A, Wang J, Abbate M, Zoja C, Remuzzi G. Insulin-like growth factor-1 sustains stem cell mediated renal repair. J Am Soc Nephrol. 2007;18:2921–2928. doi: 10.1681/ASN.2006121318. [DOI] [PubMed] [Google Scholar]
- 12.Dekel B, Shezen E, Even-Tov-Friedman S, Katchman H, Margalit R, Nagler A, Reisner Y. Transplantation of human hematopoietic stem cells into ischemic and growing kidneys suggests a role in vasculogenesis but not tubulogenesis. Stem Cells. 2006;24:1185–1193. doi: 10.1634/stemcells.2005-0265. [DOI] [PubMed] [Google Scholar]
- 13.Basile DP. The endothelial cell in ischemic acute kidney injury: implications for acute and chronic function. Kidney Int. 2007;72:151–156. doi: 10.1038/sj.ki.5002312. [DOI] [PubMed] [Google Scholar]
- 14.Basile DP. Challenges of targeting vascular stability in acute kidney injury. Kidney Int. 2008;74:257–258. doi: 10.1038/ki.2008.243. [DOI] [PubMed] [Google Scholar]
- 15.Basile DP, Donohoe D, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol. 2001;281:F887–899. doi: 10.1152/ajprenal.2001.281.5.F887. [DOI] [PubMed] [Google Scholar]
- 16.Yuan HT, Li XZ, Pitera JE, Long DA, Woolf AS. Peritubular capillary loss after mouse acute nephrotoxicity correlates with down-regulation of vascular endothelial growth factor-A and hypoxia-inducible factor-1 alpha. Am J Pathol. 2003;163:2289–2301. doi: 10.1016/s0002-9440(10)63586-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Bohm M, Nickenig G. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 18.Dimmeler S, Zeiher AM. Vascular repair by circulating endothelial progenitor cells: the missing link in atherosclerosis? J Mol Med. 2004;82:671–677. doi: 10.1007/s00109-004-0580-x. [DOI] [PubMed] [Google Scholar]
- 19.Schatteman G. Are circulating CD133+ cells biomarkers of vascular disease? Arterioscler Thromb Vasc Biol. 2005;25:270–271. doi: 10.1161/01.ATV.0000154484.58485.24. [DOI] [PubMed] [Google Scholar]
- 20.Bertolini F, Shaked Y, Mancuso P, Kerbel RS. The multifaceted circulating endothelial cell in cancer: towards marker and target identification. Nat Rev Cancer. 2006;6:835–845. doi: 10.1038/nrc1971. [DOI] [PubMed] [Google Scholar]
- 21.Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9:702–712. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- 22.Taguchi A, Soma T, Tanaka H, Kanda T, Nishimura H, Yoshikawa H, Tsukamoto Y, Iso H, Fujimori Y, Stern DM, Naritomi H, Matsuyama T. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004;114:330–338. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tei K, Matsumoto T, Mifune Y, Ishida K, Sasaki K, Shoji T, Kubo S, Kawamoto A, Asahara T, Kurosaka M, Kuroda R. Administrations of peripheral blood CD34-positive cells contribute to medial collateral ligament healing via vasculogenesis. Stem Cells. 2008;26:819–830. doi: 10.1634/stemcells.2007-0671. [DOI] [PubMed] [Google Scholar]
- 24.Romagnani P, Annunziato F, Liotta F, Lazzeri E, Mazzinghi B, Frosali F, Cosmi L, Maggi L, Lasagni L, Scheffold A, Kruger M, Dimmeler S, Marra F, Gensini G, Maggi E, Romagnani S. CD14+CD34low cells with stem cell phenotypic and functional features are the major source of circulating endothelial progenitors. Circ Res. 2005;97:314–322. doi: 10.1161/01.RES.0000177670.72216.9b. [DOI] [PubMed] [Google Scholar]
- 25.Elsheikh E, Uzunel M, He Z, Holgersson J, Nowak G, Sumitran-Holgersson S. Only a specific subset of human peripheral-blood monocytes has endothelial-like functional capacity. Blood. 2005;106:2347–2355. doi: 10.1182/blood-2005-04-1407. [DOI] [PubMed] [Google Scholar]
- 26.Furuichi K, Gao JL, Murphy PM. Chemokine receptor CX3CR1 regulates renal interstitial fibrosis after ischemia-reperfusion injury. Am J Pathol. 2006;169:372–387. doi: 10.2353/ajpath.2006.060043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, Collins AJ. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol. 2009;20:223–228. doi: 10.1681/ASN.2007080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim J, Seok YM, Jung KJ, Park KM. Reactive oxygen species/oxidative stress contributes to progression of kidney fibrosis following transient ischemic injury in mice. Am J Physiol Renal Physiol. 2009 doi: 10.1152/ajprenal.90735.2008. [DOI] [PubMed] [Google Scholar]
- 29.Blann AD, Woywodt A, Bertolini F, Bull TM, Buyon JP, Clancy RM, Haubitz M, Hebbel RP, Lip GY, Mancuso P, Sampol J, Solovey A, Dignat-George F. Circulating endothelial cells. Biomarker of vascular disease. Thromb Haemost. 2005;93:228–235. doi: 10.1160/TH04-09-0578. [DOI] [PubMed] [Google Scholar]
- 30.Lin SL, Castano AP, Nowlin BT, Lupher ML, Jr, Duffield JS. Bone marrow Ly6Chigh monocytes are selectively recruited to injured kidney and differentiate into functionally distinct populations. J Immunol. 2009;183:6733–6743. doi: 10.4049/jimmunol.0901473. [DOI] [PubMed] [Google Scholar]
- 31.Bagshaw SM. Short- and long-term survival after acute kidney injury. Nephrol Dial Transplant. 2008;23:2126–2128. doi: 10.1093/ndt/gfn300. [DOI] [PubMed] [Google Scholar]
- 32.Matsumoto T, Kawamoto A, Kuroda R, Ishikawa M, Mifune Y, Iwasaki H, Miwa M, Horii M, Hayashi S, Oyamada A, Nishimura H, Murasawa S, Doita M, Kurosaka M, Asahara T. Therapeutic potential of vasculogenesis and osteogenesis promoted by peripheral blood CD34-positive cells for functional bone healing. Am J Pathol. 2006;169:1440–1457. doi: 10.2353/ajpath.2006.060064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gaia S, Smedile A, Omede P, Olivero A, Sanavio F, Balzola F, Ottobrelli A, Abate ML, Marzano A, Rizzetto M, Tarella C. Feasibility and safety of G-CSF administration to induce bone marrow-derived cells mobilization in patients with end stage liver disease. J Hepatol. 2006;45:13–19. doi: 10.1016/j.jhep.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 34.Van Epps DE, Bender J, Lee W, Schilling M, Smith A, Smith S, Unverzagt K, Law P, Burgess J. Harvesting, characterization, and culture of CD34+ cells from human bone marrow, peripheral blood, and cord blood. Blood Cells. 1994;20:411–423. [PubMed] [Google Scholar]
- 35.Weisel KC, Moore MA, Kanz L, Mohle R. Extended in vitro expansion of adult, mobilized CD34+ cells without significant cell senescence using a stromal cell coculture system with single cytokine support. Stem Cells Dev. 2009;18:229–234. doi: 10.1089/scd.2008.0069. [DOI] [PubMed] [Google Scholar]
- 36.Woywodt A, Blann AD, Kirsch T, Erdbruegger U, Banzet N, Haubitz M, Dignat-George F. Isolation and enumeration of circulating endothelial cells by immunomagnetic isolation: proposal of a definition and a consensus protocol. J Thromb Haemost. 2006;4:671–677. doi: 10.1111/j.1538-7836.2006.01794.x. [DOI] [PubMed] [Google Scholar]
- 37.Timmermans F, Plum J, Yoder MC, Ingram DA, Vandekerckhove B, Case J. Endothelial progenitor cells: identity defined? J Cell Mol Med. 2009;13:87–102. doi: 10.1111/j.1582-4934.2008.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yeh ET, Zhang S, Wu HD, Korbling M, Willerson JT, Estrov Z. Transdifferentiation of human peripheral blood CD34+-enriched cell population into cardiomyocytes, endothelial cells, and smooth muscle cells in vivo. Circulation. 2003;108:2070–2073. doi: 10.1161/01.CIR.0000099501.52718.70. [DOI] [PubMed] [Google Scholar]
- 39.Newman PJ. The biology of PECAM-1. J Clin Invest. 1997;100:S25–29. [PubMed] [Google Scholar]
- 40.Asahara T, Kawamoto A. Endothelial progenitor cells for postnatal vasculogenesis. Am J Physiol Cell Physiol. 2004;287:C572–579. doi: 10.1152/ajpcell.00330.2003. [DOI] [PubMed] [Google Scholar]
- 41.Matsumoto T, Kuroda R, Mifune Y, Kawamoto A, Shoji T, Miwa M, Asahara T, Kurosaka M. Circulating endothelial/skeletal progenitor cells for bone regeneration and healing. Bone. 2008;43:434–439. doi: 10.1016/j.bone.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 42.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 43.Kollet O, Shivtiel S, Chen YQ, Suriawinata J, Thung SN, Dabeva MD, Kahn J, Spiegel A, Dar A, Samira S, Goichberg P, Kalinkovich A, Arenzana-Seisdedos F, Nagler A, Hardan I, Revel M, Shafritz DA, Lapidot T. HGF, SDF-1, and MMP-9 are involved in stress-induced human CD34+ stem cell recruitment to the liver. J Clin Invest. 2003;112:160–169. doi: 10.1172/JCI17902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duffield JS, Park KM, Hsiao LL, Kelley VR, Scadden DT, Ichimura T, Bonventre JV. Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J Clin Invest. 2005;115:1743–1755. doi: 10.1172/JCI22593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lombardi D, Gordon KL, Polinsky P, Suga S, Schwartz SM, Johnson RJ. Salt-sensitive hypertension develops after short-term exposure to Angiotensin II. Hypertension. 1999;33:1013–1019. doi: 10.1161/01.hyp.33.4.1013. [DOI] [PubMed] [Google Scholar]
- 46.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 47.Freedman SB, Isner JM. Therapeutic angiogenesis for coronary artery disease. Ann Intern Med. 2002;136:54–71. doi: 10.7326/0003-4819-136-1-200201010-00011. [DOI] [PubMed] [Google Scholar]
- 48.Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 176:85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin SL, Kisseleva T, Brenner DA, Duffield JS. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol. 2008;173:1617–1627. doi: 10.2353/ajpath.2008.080433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomas SE, Anderson S, Gordon KL, Oyama TT, Shankland SJ, Johnson RJ. Tubulointerstitial disease in aging: evidence for underlying peritubular capillary damage, a potential role for renal ischemia. J Am Soc Nephrol. 1998;9:231–242. doi: 10.1681/ASN.V92231. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.