Abstract
The question of whether T cell responses to SEREX-defined tumor antigens are under regulation of naturally occurring CD4+CD25+ regulatory T cells (nTreg cells) has not been answered. To address this issue, we first identified an HLA-A2.1-restricted T cell antigen epitope of SEREX-identified tumor antigen CML66L, 66Pa. The HLA-A2.1/66Pa peptide complex in vitro stimulated the in vivo-primed T cells as shown by increased T cell proliferation, higher secretion of the T cell cytokine interferon-γ (IFN-γ), increased production of intracellular IFN-γ in CD8+ T cells, and higher T cell-mediated cytotoxicities of CML66L+ human tumor cells. This suggests that CML66L elicits T cell immune responses. We also developed a novel internal reference epitope for identification of T cell epitopes by construction of chimeric CML66L containing myeloid antigen proteinase 3 epitope Pr1 as a control. Finally, we found that nTreg cells regulates T cell responses to 66Pa, and that depletion of nTreg cells via a pro-apoptotic protein Bax-dependent mechanism enhances polyclonal T cell responses to 66Pa. These findings provide new insights into the T cell participation in SEREX-defined anti-tumor immune responses and novel direction in enhancement of anti-leukemia immunotherapy by modulation of homeostasis of nTreg cells.
Keywords: leukemia antigen CML66L, HLA-A2.1-restriction, T cell antigen epitope, CD4+CD25+ regulatory T cells, SEREX antigens, apoptosis
Despite advances in the therapy of leukemia, most patients will die from their disease. Therefore, new therapeutics are being actively pursued (1–2). Adoptive immunotherapy with donor T cells (DLI) can provide long-lasting remission provides compelling evidence that these cells play an important role in mediating a graft-versus-leukemia (GVL) response after allogeneic bone marrow transplantation (allo-BMT) (3). However, the antigens mediating the anti-leukemia immune response remain unknown (4). By using a serological cloning (SEREX) approach (4), we identified a novel antigen, CML66L, also termed as NudC domain containing 1 (66 kD) (5). CML66L is up-regulated in chronic myeloid leukemia (CML) cells and in a variety of tumors but is not expressed in normal tissues except testis (5–6), which is similar to that of cancer-testis antigens (7). Alternative splicing is a key mechanism for the enhanced immunogenicity of this antigen (8). IgG responses to CML66L are found in patients with various tumors, suggesting that CML66L is broadly immunogenic (5). Furthermore, IgG responses to CML66L are temporally associated with CML remission induced by DLI (5), implying that CML66L-specific IgG and T cells contribute to antileukemic responses.
The IgG and T cell responses can be regulated integrally or independently (9). In order to define the potential of SEREX antigens in elicitation of T cell responses (10), we took CML66L as a model leukemia antigen in identification of HLA-A2.1-restricted T cell antigen epitopes (11–12). In addition, immune tolerance may play a role in suppressing anti-CML66L responses in patients who lacked the CML66L antibody (5). Therefore, in this study, we have also addressed two important issues, including whether immune suppression to the CML66L are regulated by naturally occurring CD4+CD25+CD62+ regulatory T cells (nTreg cells) (13–14), and whether homeostasis of Treg cells affects immune responses to CML66L. These findings provide new insights into the integrated T cell and IgG anti-tumor responses and novel direction in enhancement of anti-tumor immunotherapy by modulation of homeostasis of nTreg cells (2).
MATERIALS AND METHODS
Mice
HLA-A2.1/Kb mice were provided by Dr. L. Sherman at the Scripps Institute (15). The immunization was performed as described (16) and approved by the IRB of Temple University.
DNA Vaccination
CML66L cDNA was inserted in the EcoRI of the plasmid pSINCP, which was provided by Dr. J. Polo (Chiron Technologies) (16). A pSINCP-β-galactosidase plasmid was used as a control. The reported HLA-A2.1-restricted proteinase 3 epitope Pr1 was engineered into a CML66L region (aa 283–291) in pSINCP-CML66L using a Site-directed Mutagenesis Kit (Stratagene, La Jolla, CA) with two mutagenic primers. The sense primer Pr1A (5′-GAACCTCTGTATTACTGGATCCAGGTTTTACAAGAATTGAATGTAACCGTACGGCTT CCAGAAGACA-3′) and the antisense primer Pr1B (5′-TGTCTTCTGGAAGCCGTACGGTTACATTCAATTCTTGTAAAACCTGGATCCAGTAATACAGAGGTTC-3′) were synthesized. The underlined sequences were designed for mutation. A solution of 0.1 ml containing 100 μg of plasmid DNA (pSINCP-CML66L, pSINCP-β-galactosidase, or pSINCP-CML66L-Pr1) formulated with a local anesthetic (0.25% bupivacaine; Sigma, St. Louis, MO) was injected intramuscularly into the quadriceps of the mice. At the time points indicated in Fig. 2, blood samples were collected from the tail vein.
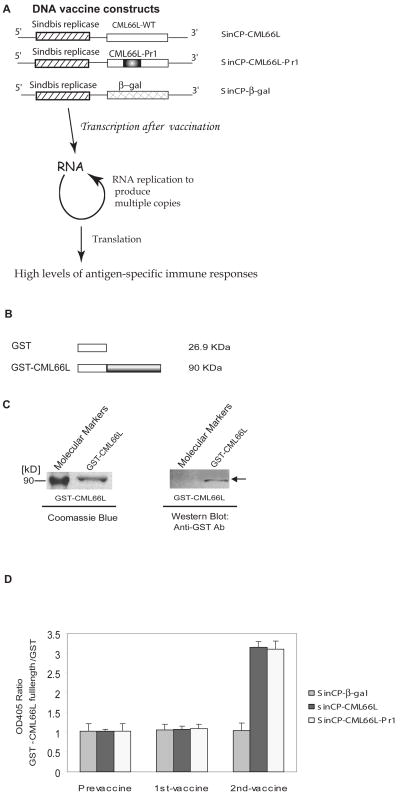
Fig. 2. CML66L elicits IgG antibody responses in HLA-A2.1/Kb transgenic mice after CML66L DNA vaccination.
A. DNA vaccine constructs presented in the upper panel. CML66L cDNA, CML66L-Pr1 cDNA, and β-galactosidase cDNA were subcloned into the pSINCP vector with the Sindbis viral replicon. In the lower panel, the advantage of the high vaccination efficiency of the pSinCP vector in achieving high levels of antigen-specific immune responses is schematically presented. B. Glutathione S-transferase (GST) fusion protein construct of CML66L. The full-length cDNA of CML66L was fused in frame into the C-terminus of GST in the pGEX-3X vector. C. High purity of GST-CML66L fusion protein. The high purity of the purified GST-CML66L was examined with SDS-PAGE followed by staining with Coomassie blue, and the specificity of the purified GST-CML66L was confirmed with Western blotting using anti-GST antibodies, which did recognize the GST-CML66L band (indicated by an arrow) but did not recognize the molecular marker band. D. The specific antibody responses to CML66L and CML66L-Pr1 in vaccinated HLA-A2.1/Kb transgenic mice. The specific antibody responses to CML66L in mouse serum samples were detected by ELISA with purified GST and GST-CML66L as the plate-coating antigens. As described in Materials and Methods, only six of the 583 amino acids in CML66L were mutated to engineer the epitope Pr1 of control antigen proteinase 3 into a position (aa 283-aa 291) on CML66L; thus, the antibody responses to CML66L-Pr1 were examined using GST-CML66L as the ELISA coating antigen, the same as in the examination of anti-CML66L antibody responses with ELISA. The OD405 ratio for each sample was calculated as the OD405 reading of CML66L normalized by the OD405 reading of GST. The ELISA experiment was repeated three times for each sample, and the mean and standard deviation were calculated and presented.
Tumor Cells
Human carcinoma HeLa cells and myeloid leukemia K562 cells were transfected with a plasmid containing HLA-A2.1 cDNA (provided by Drs. T. Boon, and P.G. Coulie) (17). The increased expression of HLA-A2.1 in the cell surface was evaluated using FITC-conjugated HLA-A2.1 and isotype (BD Pharmingen, San Diego, CA) by FACS.
Antigenic Peptides
The prediction of proteasome cleavage sites and the peptides binding to the HLA-A2.1 molecule was performed as we reported (18). Two out of three high score peptides were synthesized, peptide 66Pa (306-FLPDHINIV-314) and peptide 66Pb (568-VLTTKNLFL-576) (Figs. 1A and 1B) with the purity (>95%) (Sigma-Genosys, Woodlands, TX). The peptide 66N1(308-PDHINIVLK-316) was synthesized as a negative control. In addition, the positive control Pr1 from tumor antigen proteinase 3 (169-VLQELNVTV-177) was synthesized as reported (Figs. 1A and 1B) (19).
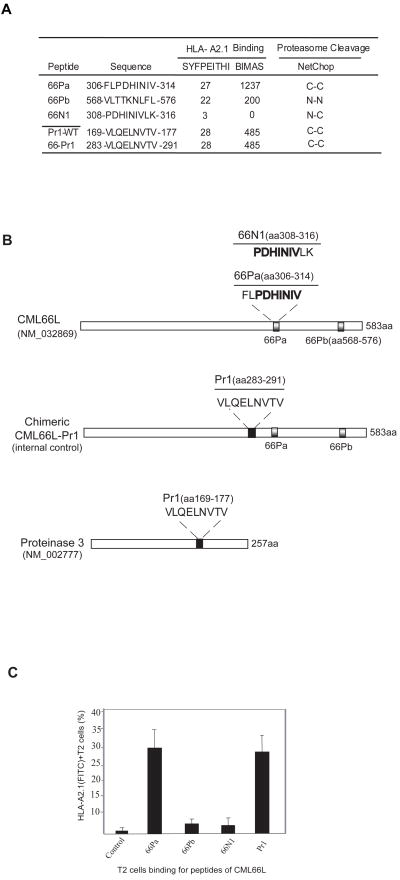
Fig. 1. CML66L encodes a high-affinity HLA-A2.1-binding epitope.
A. Epitope prediction on the basis of potential for HLA-A2.1 binding and proteasome cleavage. Five epitope and epitope candidates are presented. The HLA-A2.1 binding scores predicted by the algorithms SYFPEITHI and BIMAS are presented. The epitope sequences are listed with the starting position and ending position in tumor antigens. The prediction of the N-terminal cleavage site of the candidate epitopes is separated with “-” from the prediction of the C-terminal cleavage site of the epitopes, using the NetChop algorithm. “C” indicates that there is a site for cleavage; “N” indicates that there are no sites for cleavage. Pr1 is transplanted by site-directed mutagenesis from the proteasome cleavage-favorable position “C-C” in wild-type proteinase 3 (GenBank Accession Number NM_002777) to the proteasome-favorable position “C-C” in chimeric antigen CML66L-Pr1. B. The relative positions of the epitopes 66Pa, 66Pb, and 66N1 in wild-type CML66L (GenBank Accession Number NM_032869) and the chimeric antigen CML66L-Pr1, and the relative position (aa 169-aa 177) of Pr1 in wild-type tumor antigen proteinase 3. C. The HLA-A2.1-stabilizing potential of CML66L epitopes. A T2 cell assay was used to measure the HLA-A2.1-stabilizing potential of the candidate epitopes of CML66L and the positive control, Pr1. The stabilizing potentials were quantified by the percentage of HLA-A2.1+ T2 cells detected by flow cytometry with an anti-HLA-A2.1 antibody.
T2 Cell-Binding Assay
After incubation of T2 cells (ATCC, Manassas, VA) with the peptides (100 μg/ml) and exogenous β2 molecules (3 μg/ml; Sigma), the surface expression of HLA-A2.1was assayed by FACS with FITC-conjugated HLA-A2.1 antibodies (19).
Generation and Purification of Glutathione S-Transferase (GST) Fusion Protein
The purification of GST and GST-CML66L were performed as reported (5).
Detection of CML66L-Specific IgG Antibodies in Sera from immunized mice by ELISA
ELISA was performed as we described previously (5).
T Cell Proliferation Assay
4 × 105 splenic T cells from immunized mice were activated with antigenic peptides in triplicate (20). After five days of T cell stimulation, 1 μCi of [3H]thymidine (GE Healthcare) incorporation were measured in a Scintillation Counter (Perkin-Elmer, Boston, MA).
Secreted Interferon-γ (IFN-γ) Assay and Intracellular IFN-γ Staining
To investigate whether T cell recognition of antigen peptides was HLA-A2.1-dependent, a T cell interferon-γ (IFN-γ) secretion assay (21) was performed using a mouse IFN-γ BD Opt EIA ELISA kit (BD Pharmingen) in the absence or presence of HLA-A2.1 blocking antibodies (BD Pharmingen). For determination of the cell type of IFN-γ production, intracellular IFN-γ staining of CD8+ T cells was performed (BD Pharmingen) (22).
Cytotoxic T Lymphocyte (CTL) Assay
The CTL activity was measured using a cytotoxicity assay (Promega, Madison WI) on the plate reader (Molecular Devices). The normalized values were computed for the percent cytotoxicity for each effector-target cell ratio: % cytotoxicity = [(Experimental − Effector Spontaneous − Target Spontaneous)/(Target Maximum − Target Spontaneous)] × 100.
Construction of CD25+ T Cell-Targeting Mouse IL-2Rα Promoter-Bax Vector
Mouse CD25 promoter −2539 to +93 (GenBank: M16398) vector was provided by P. Reichenbach and M. Nabholz (23). The vector pcDNA3-mouse Bax (GenBank: NM_007527) was provided by Dr. J.P. Zha. The CMV promoter was removed by with two sites (Nru I and Hind III), and replaced by a short linker (NruI – Sma I – Hpa I – Hind III), which was generated by oligonucleotides NSH16 (5′-CGACCCGGGGTTAACA-3 ′) and NSH20 (5′-AGCTTGTTAACCCCGGGTCG-3′). The new pCD25 vector was constructed by subcloning the CD25 promoter (a HindIII-EcoRI fragment) into the HindIII and EcoRI sites in the promoter-free pcDNA3 backbone. For detection of transfected Bax rather than endogenous Bax, a short c-Myc expression tag (13 aa: MASMQKLISEEDL) (24) was fused into the N-terminus of Bax. A cDNA encoding Kozak consensus-c-Myc tag-Bax was generated by high-fidelity PCR using two oligonucleotide primers, Bax myc5 (5′-CCGGGAATTCACCATGGCATCAATGCAGAAGCTGATCTCAGAGGAGGACCTGATGGACGGGTCCGGGGAGCAGCTT-3′) and Bax myc3 (5′-CCGGGAATTCTCAGCCCATCTTCTTCCAGATGGT-3′), followed by subcloning into the EcoRI of the new pCD25 vector.
Transfection of Primary T cells and Assay for T cell Apoptosis
Mouse T cell CTLL2 (ATCC) and mouse splenic cells were transfected with CD25+ regulatory T cell-targeted vector pCD25-Bax and pCD25 control vector, respectively, using the Amaxa Kit (Amaxa Biosystems, Cologne, Germany). Apoptosis were assayed by using an Annexin V-FITC Kit II (BD Pharmingen) in combination with PE-CD4 and PE-CY7-CD25 (BD Pharmingen) in gated live T cells (25).
Comparison of T cell responses to CML66L epitope in the presence or absence of CD4+CD25+ Treg cells, or transfected CD4+CD25+ Treg cells
Treg cells in the splenocytes of immunized mice were depleted with biotin-rat anti-CD25 antibody and a Biotin Binder Kit (Dynal Biotech, Brown Deer, WI) or purified with a CD4+CD25+ Treg Kit (Miltenyi Biotec, Auburn, CA). 2 × 106 CD4+ or CD4+CD25+ Tregs-depleted splenocytes were transfected with pCD25 promoter -Bax (pCD25-Bax) or pCD25 promoter control vector (pCD25 vector) with Amaxa Nucleofector Kit (Amaxa). Next, 1 × 104 CD4+CD25+ cells were either left untransfected, transfected with pCD25-Bax, or transfected with pCD25 vector and then added to cells with 5 μg/ml 66Pa with the Fig. 6A group settings in Fig. 6B. After being incubated for 5 days, 66Pa- and Pr1-pulsed splenocytes were irradiated. After being washed twice, irradiated cells were added to each group and cultured for an additional 15 hours. Intracellular IFN-γ staining was performed using a Cytofix/Cytoperm kit (BD Pharmingen) according to the manufacturer’s instructions.
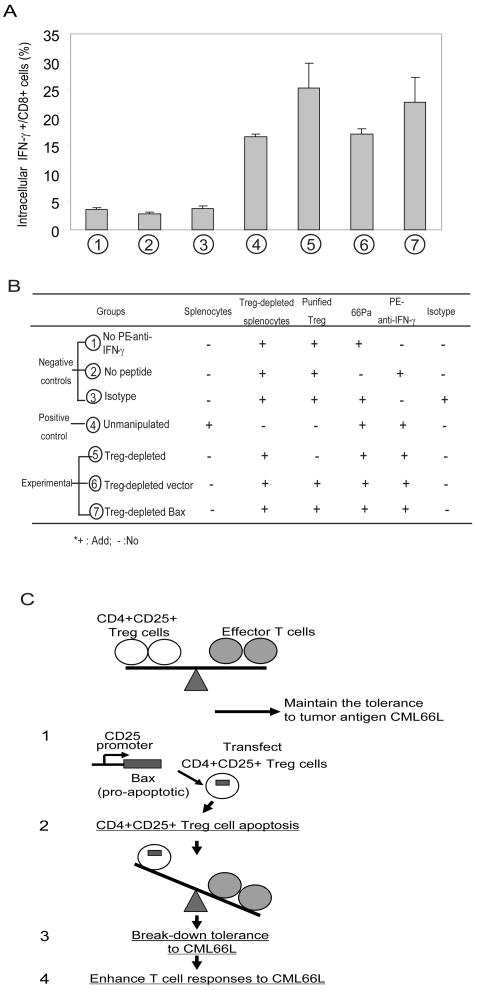
Fig. 6. Increased apoptosis of CD4+CD25+ Treg cells via a Bax-dependent mechanism enhances T cell immune responses to CML66L.
A. The enhancement of intracellular IFN-γ production in CD8+ T cells after restimulation in vitro with 66Pa in the presence or absence of CD4+CD25+ Treg cells by either depletion using anti-CD25 antibodies or transfection with CD25 promoter-c-myc-Bax. The seven groups are described in Fig. 6B. The percentages of intracellular IFN-γ+CD8+ T cells in each group are presented. The experiments presented were repeated three times. The mean and standard deviation were calculated for each group. B. The group settings for the experiments presented in Fig. 6A. Four groups were used as controls (negative or positive) including three negative control groups (Figs. 6A): (1) no-antibody staining control composed of Treg cell-depleted splenocytes reconstituted with purified Treg cells and 66Pa but not stained with PE-conjugated anti-IFN-γ antibodies; (2) no-peptide cell control composed of Treg cell-depleted splenocytes reconstituted with purified Treg cells without the addition of 66Pa; (3) PE-Ig isotype control composed of Treg cell-depleted splenocytes reconstituted with purified nTreg cells and 66Pa and stained by PE-antibody isotype control; and (4) one positive control of un-manipulated splenocytes composed of splenocytes stimulated by 66Pa and stained with PE-conjugated anti-IFN-γ antibodies. Three experimental groups were set up (Figs. 6A): (5) nTreg cell-depleted group composed of Treg cell-depleted splenocytes with 66Pa stimulation and stained with PE-conjugated anti-IFN-γ antibodies; (6) vector control-transfected nTreg cells composed of nTreg cell-depleted splenocytes with 66Pa stimulation and with reconstitution of nTreg cells transfected by vector control, and stained with PE-conjugated anti-IFN-γ antibodies; (7) Bax-transfected Treg cells composed of nTreg cell-depleted splenocytes with 66Pa stimulation and with reconstitution of nTreg cells transfected by Bax cDNA, and stained with PE-conjugated anti-IFN-γ antibodies. C. The schematic representation of a novel working model: Depletion of CD4+CD25+ Treg cells via a Bax-dependent mechanism enhances T cell responses to self-tumor antigen CML66L. The upper panel suggests that CD4+CD25+ Treg cells play a role in maintaining tolerance to self-tumor antigen CML66L. The lower panel recapitulates the mechanism and results presented in Fig. 6A, suggesting that increased apoptosis of CD4+CD25+ Treg cells via a Bax-dependent mechanism enhances T cell immune responses to CML66L.
RESULTS
CML66L encodes a high-affinity HLA-A2.1-binding epitope, 66Pa
We first examined the potential of CML66L in encoding high affinity HLA-A2.1 binding epitopes. As shown in Fig. 1A, CML66L encoded two peptides that have a high SYFPEITHI HLA-A2.1 binding potential, 66Pa (score, 27) and 66Pb (score, 22). In addition, 66Pa also had a higher BIMAS score (1237) than that of 66Pb (200). Both ends of peptide 66Pa had good potential to be cleaved by proteasomes predicted by the algorithm NetChop. In contrast, NetChop predicted that peptides 66Pb and 66N1 could not be cleaved by proteasomes. We found that it is useful to include the algorithm for proteasome cleavage in addition to the algorithms for MHC binding. Consistent with our findings, others also have suggested that if peptide epitopes with high binding potential for the MHC allele are located in the sequence context that cannot be cleaved by proteasome, it is less likely that the epitopes are immunodominant (18). Our analysis also included documented myeloid tumor antigen proteinase 3 epitope Pr1 as a positive control (19). Pr1 had a BIMAS score of 485 and a SYFPEITHI score of 28, and NetChop predicted that it was cleavable (Figs. 1A and 1B). Thus, the scores for 66Pa were comparable to those for Pr1, suggesting that 66Pa is a promising HLA-A2.1-restricted T cell antigen epitope.
To examine the binding capability of CML66L peptides, we performed the T2 cell HLA-A2.1-binding assay (19). The percentage of HLA-A2.1 expression after pulsing with epitope peptides in the presence of exogenous β2 molecules was used as an in vitro index of their HLA-A2.1 binding affinity in the stimulation of HLA-A2.1-restricted CD8+ T cell responses. The T2 cell HLA-A2.1-binding assay showed that 66Pa had the highest potential for binding to HLA-A2.1 (29.6% ± 5.1%) among the CML66L peptides (Fig. 1C). Pr1, the positive control, had 28.2% ± 5.3% potential for HLA-A2.1 binding in our experiments (Figs. 1B and 1C), similar to that previously reported (19). Thus, 66Pa had a binding capacity similar to that of Pr1, suggesting that 66Pa is the dominant HLA-A2.1-binding epitope among the putative HLA-A2.1 peptides of CML66L. Although 66Pb had high scores predicted by both algorithms, its overall ability to stabilize the expression of the HLA-A2.1 complex on the T2 cell surface was low (2.65% ± 2.72%; Fig. 1C). In addition, the algorithm predicted that 66Pb had no complete sites for cleavage by proteasomes (Fig. 1A). Therefore, we focused on identifying 66Pa as the candidate epitope of CML66L in the subsequent experiments. Furthermore, as expected, 66N1, which had the lowest HLA-A2.1-binding scores (Figs. 1A and 1B), also had a low binding score on the T2 cell assay (1.96 ± 0.22; Fig. 1C) and was thus chosen as the negative control peptide. Of note, the results of T2 cell binding stayed the same using either the percentage of positive cells (55) or the mean fluorescence intensity (MFI) (not shown).
CML66L can elicit IgG antibody and T cell responses in HLA-A2.1/Kb transgenic mice after CML66L DNA vaccination
We reasoned that overexpression of human CML66L by vaccinating mice with DNA (16) could overcome the tolerance for the highly homologous mouse CML66L protein (90.4%) (8), and lead to immune responses to human CML66L. To investigate this possibility, we vaccinated HLA-A2.1 transgenic mice (15) with the vector pSINCP containing either CML66L cDNA, CML66L-Pr1, or β-galactosidase (Fig. 2A). The rationale in application of this plasmid was that (Fig. 2A) the plasmid contains a Sindbis virus replicon that produces a large amount of self-replicating RNA and leads to much higher expression of the introduced protein (16). The advantage in using HLA-A2.1/Kb transgenic mice was that vaccinating them allowed the identification of polyclonal activated T cells with HLA-A2.1 restriction on an amplified scale (15). By using ELISA with diluted sera (1:500) (15) (Figs. 2B and 2C), we found that vaccination of HLA-A2.1/Kb transgenic mice, followed by boosting with either plasmid DNA pSINCP-CML66L or pSINCP-CML66L-Pr1 led to increased titer of IgG antibody to CML66L (Fig. 2D). In contrast, vaccination with the DNA pSINCP-β-galactosidase, a negative control, did not elicit antibody responses to CML66L (Figs. 2A and 2D), suggesting that immune responses to CML66L are specific. Of note, elicitation of high titer IgG antibody responses after vaccination suggested that helper T cells are participated in anti-CML66L antibody responses since T helper cell function is required for high titer IgG responses (9). In addition, vaccination with two additional expression vectors containing CML66L cDNA (pcDNA5-CML66L and pSec-CML66L) did not elicit detectable IgG antibody responses (data not shown). These findings suggested that pSINCP-CML66L with the RNA replicon is much more efficient than other conventional expression vectors as a DNA vaccination vector in HLA-A2.1/Kb transgenic mice (16). Of note, our ELISA also provided direct evidence that chimeric pSINCP-CML66L-Pr1 has the same efficiency as pSINCP-CML66L in inducing a CML66L antigen-specific immune response. Therefore, in the following experiments we used pSINCP-CML66L-Pr1 in DNA vaccination in mice so that the positive control epitope Pr1 would be included.
For characterization of the ability of 66Pa to stimulate T cells in vitro, T cells were isolated from DNA-vaccinated HLA-A2.1/Kb transgenic mice and stimulated in vitro with 66Pa pulsed on irradiated HLA-A2.1-transfected HeLa cells for 5 days. We found that 66Pa did stimulate T cell proliferation, as shown by [3H]thymidine incorporation (Fig. 3A), more than Pr1 did (19). In addition, as expected, neither 66N1 nor the cell control stimulated significant T cell proliferation (Fig. 3A). Of note, neither 66Pa nor Pr1 stimulated T cell proliferation in T cell preparations from unvaccinated HLA-A2.1/Kb mice (data not shown), suggesting that 66Pa was processed from the immunized CML66L in the antigen-presenting cells in vaccinated HLA-A2.1/Kb transgenic mice.
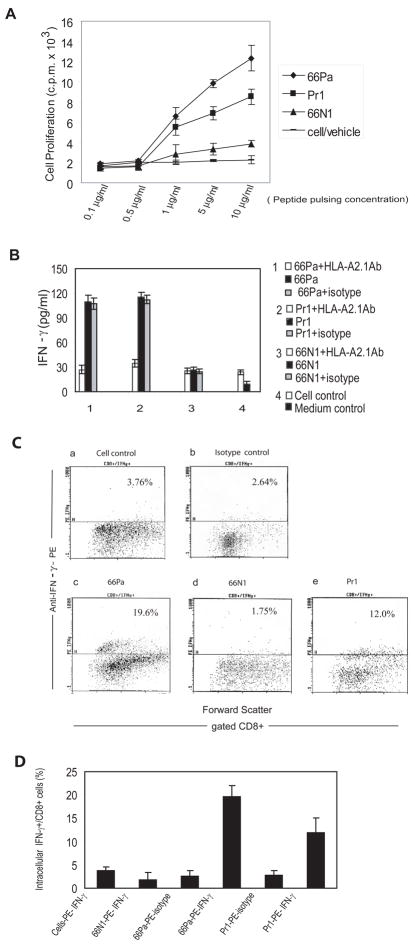
Fig. 3. CML66L elicits HLA-A2.1-restricted T cell responses in HLA-A2.1/Kb transgenic mice after DNA vaccination.
A. T cell proliferation following in vivo priming and in vitro restimulation with the epitope peptides. The T cells were isolated from pSinCP-CML66L-Pr1-vaccinated mice and restimulated in vitro with the epitope peptides in peptide pulsing concentrations of 0.1, 0.5, 1, 5, and 10μg/ml. T cell proliferation was measured by incorporation of [3H]thymidine and presented as the c.p.m. × 103. B. IFN-γ secretion from T cells primed in vivo after in vitro restimulation with the epitope peptides. IFN-γ secretion was measured after in vitro restimulation of T cells with the epitope peptides in the presence or the absence of HLA-A2.1-blocking antibody or antibody isotype blocking control for 66Pa, the positive control epitope Pr1, and the negative control peptide 66N1. C. The flow cytometric dot plot of the intracellular IFN-γ production in CD8+ T cells after in vitro restimulation of T cells with the epitope peptides. a) No stimulation control: Intracellular IFN-γ was measured in the gated CD8+ T cells without in vitro peptide stimulation and assayed by anti-IFN-γ antibodies; b) Stimulation with 66Pa measured by antibody isotype control: Intracellular IFN-γ was measured in gated CD8+ T cells after in vitro stimulation with 66Pa and assayed by antibody isotype control; c) Stimulation with 66Pa measured by anti-IFN-γ: Intracellular IFN-γ was measured in gated CD8+ T cells after in vitro stimulation with 66Pa and assayed by anti-IFN-γ antibodies; d) Stimulation with the negative control peptide 66N1 measured by anti-IFN-γ antibodies: Intracellular IFN-γ was measured in gated CD8+ T cells after in vitro stimulation with 66N1 and assayed by anti-IFN-γ antibodies; e) Stimulation with the positive control Pr1: Intracellular IFN-γ was measured in gated CD8+ T cells after in vitro stimulation with the positive control peptide Pr1 and assayed by anti-IFN-γ antibodies. D. Summary of the triplicate measurements of intracellular IFN-γ production in CD8+ T cells after in vitro restimulation of T cells with the epitope peptides. The mean and standard deviation in each group are presented.
By performing an IFN-γ secretion assay, we found that 66Pa did stimulate the production of IFN-γ by expanded splenic T cells in an HLA-A2.1-restricted manner (Fig. 3B). In the controls, HLA-A2.1-restricted IFN-γ secretion by splenic T cells was also stimulated by positive control Pr1 but not by 66N1. Our findings confirmed that IFN-γ secretion by CD66L-expressing HeLa cells transfected with HLA-A2.1 cDNA was inhibited by anti-HLA-A2.1 antibody but not by the Ig isotype control. Because IFN-γ can be secreted by three types of cells, the natural killer cells, CD4+ type 1 T helper cells and CD8+ T cells (22), direct identification of IFN-γ produced by CD8+ T cells by intracellular staining was required to demonstrate MHC class I presentation of 66Pa. Stimulation by 66Pa did increase the percentage (19.60%) of CD8+ T cells producing intracellular IFN-γ over the basal level (3.76%) seen in the control cells (Figs. 3C and 3D). Similarly, Pr1 stimulation increased the percentage of CD8+ T cells to 12.00%. Identification of CML66L-specific polyclonal T cells isolated from HLA-A2.1/Kb transgenic mice vaccinated with CML66L DNA suggests that CD8+ T cells were successfully activated by the HLA-A2.1/66a peptide complex.
HLA-A2.1-restricted CML66L epitope 66Pa is naturally processed in human tumor cells
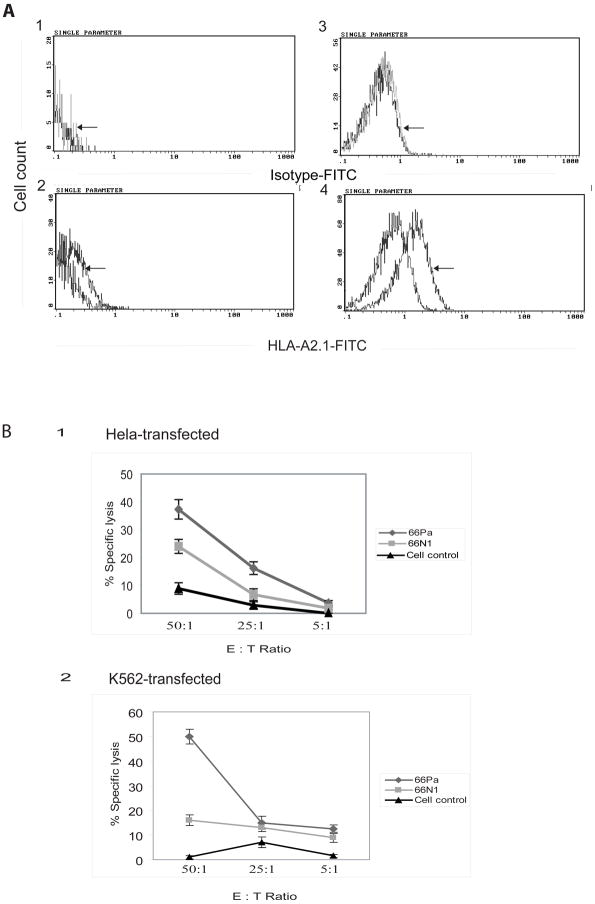
Next, we examined that whether 66Pa can be processed and presented by human tumor cells. We noticed that down-regulation of MHC molecule expression on the tumor cell surface is often a mechanism for tumors to escape host immune surveillance (1). To facilitate the detection of endogenously processed 66Pa presentation, we increased HLA-A2.1 expression in HeLa cells and K562 leukemia cells by stable transfection of these cells with HLA-A2.1 cDNA. As shown by increases in the fluorescence intensity (Figs. 4A2 and 4A4), overexpression of HLA-A2.1 induced by stable transfection with HLA-A2.1 cDNA significantly increased HLA-A2.1 expression on the cell surface. To further explore whether increased HLA-A2.1 expression in HeLa cells and K562 cells makes these transfected cells become targets for cytotoxic T cell killing, we performed a cytotoxicity assay using HLA-A2.1-transfected HeLa (Fig. 4B1) and K562 cells (Fig. 4B2) as target cells. T cells that were primed in CML66L DNA-vaccinated HLA-A2.1/Kb transgenic mice followed by in vitro restimulation with 66Pa could lyse as much as 37%-50% of the HLA-A2.1-transfected tumor cells at an effector-target cell ratio of 50:1 (Fig. 4B). The T cell cytotoxicity stimulated by 66Pa was significantly higher than that stimulated by 66N1 or without peptide stimulation (Fig. 4B). These findings demonstrated two points: first, 66Pa was naturally processed and presented in HLA-A2.1-expressing human tumor cells; second, the HLA-A2.1 peptide 66Pa presented in tumor cells mediated the cytotoxicity of mouse HLA-A2.1-restricted T cells.
Fig. 4. HLA-A2.1-restricted CML66L epitope 66Pa is naturally processed in human tumor cells.
A. Overexpression of HLA-A2.1 in HLA-A2.1 cDNA-transfected K562 leukemia cells and HLA-A2.1 cDNA-transfected HeLa cells. The increased expression of HLA-A2.1 after transfection of K562 cells and HeLa cells with HLA-A2.1 cDNA was detected (arrow) with FITC-conjugated anti-HLA-A2.1 antibodies in the HeLa cells (A2) and the K562 cells (A4) but not with FITC-conjugated antibody isotype control in the HeLa cells (A1) or the K562 cells (A3). B. The cytotoxic lyses of HLA-A2.1 cDNA-transfected K562 cells and HLA-A2.1 cDNA-transfected HeLa cells mediated by CML66L-specific T cells. The specific lyses of the transfected K562 and HeLa cells by antigen primed T cells, as shown in the percentage of specific lysis, were assayed in effector-target cell ratios of 50:1, 25:1, and 5:1. The effector T cells were primed in CML66L-vaccinated mice and restimulated in vitro with 66Pa or the negative control epitope 66N1 or were not stimulated with peptides (cell control). The experiments were performed in triplicate. The mean and standard deviation were calculated for each group.
HLA-A2.1-restricted tumor antigen proteinase 3 epitope Pr1 is engineered into the chimeric antigen CML66L-Pr1 and serves as a novel internal control in identifying T cell antigen epitopes
In the experiments for identification of T cell antigen epitopes from novel antigens, there were no references (epitopes) similar to β-actin that is commonly used as a reference for examining gene expression (8). We then investigated whether transplantation of an experimentally identified MHC class I-restricted tumor antigen epitope into a novel tumor antigen protein of interest could serve as an internal control for the identification of T cell antigen epitopes via DNA vaccination. This positive control peptide was chosen because, similar to CML66L, proteinase 3 is a tumor antigen associated with a myeloid malignancy, CML (19). To introduce minimal structural changes in the CML66L sequence and provide suitable flanking sequences with proteasome cleavage potential (18), we chose to engineer Pr1 into the CML66L location from aa 283 to aa 291 by site-directed mutagenesis to replace the CML66L sequence283TEDDLTVTI291 (mutating the underlined aa)with the Pr1 sequence 169VLQELNVTV177 (Fig. 1A and 1B). As shown in Fig. 2A and 2D, CML66L-Pr1 chimeric protein was correctly processed and retained its potential in elicitation of immune responses in vaccinated mice, similar to that of CML66L wild-type. In Fig. 3A, 3B, 3C, and 3D, our experiments showed that T cells, primed in vaccinated mice with CML66L-Pr1 chimeric DNA construct, could be further stimulated with Pr1 peptide in an HLA-A2.1 dependent manner. These results suggested that first, the Pr1 in the CML66L-Pr1 chimeric protein is correctly processed and presented to T cells and induces T cell immune responses in an HLA-A2.1-dependent manner; second, the novel SEREX antigen CML66L encodes an HLA-A2.1-restricted epitope 66Pa with an immunodominance similar to that of Pr1.
Increased apoptosis of CD4+CD25+ nTreg cells via a Bax-dependent mechanism enhances immune responses to tumor antigen CML66L
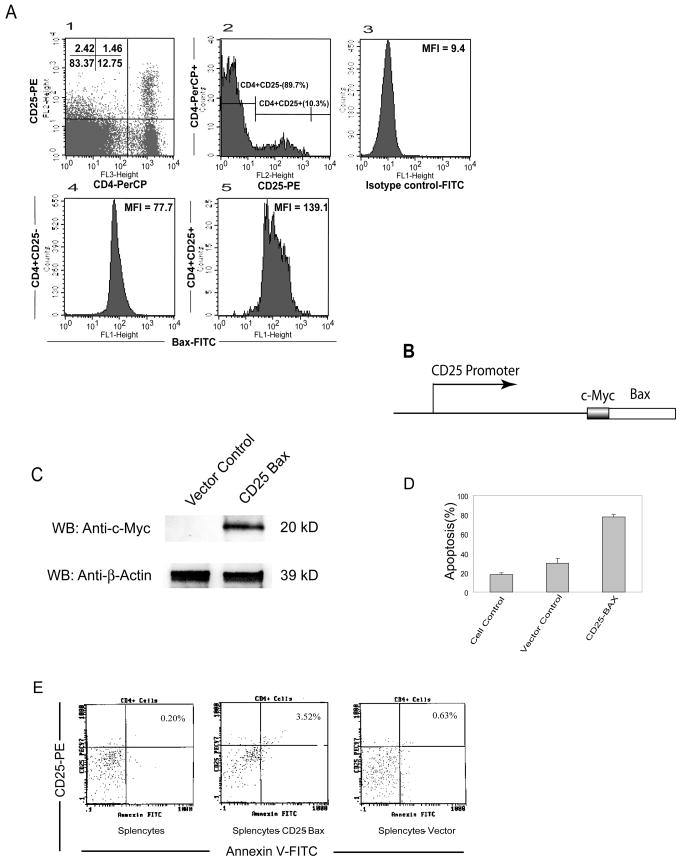
Since most identified tumor antigens including CML66L are non-mutated self-protein antigens (1), T cell self-tolerance to these self-tumor antigens is maintained by several mechanisms including one provided by nTreg cells. In addition, nTreg cells play an important role in suppression of anti-tumor immune responses once initiation occurs (14). However, it was poorly defined whether nTreg cells play any roles in the regulation of self-tumor antigen specific, HLA-A2.1-restricted T cell responses. Identification of the HLA-A2.1-restricted CML66L epitope 66Pa provided a tool to further examine this important issue. CML66L is highly expressed in proliferating acute myelogenous leukemia (AML) cells (6). However, IgG immune responses to CML66L are not detected in patients with AML (4), suggesting that anti-leukemia antigen responses may be under suppression, presumably by nTreg cells. In correlation with this point of view, nTreg cells are found to be increased in patients with AML (26). Thus, increased nTreg cells may suppress anti-tumor (leukemia) antigen T cell responses. Moreover, nTreg cells from healthy donors have higher susceptibility to apoptosis than other types of T cells (27), suggesting that nTreg cells have specific, undefined apoptosis pathways for their homeostasis. However, whether a pro-apoptotic protein prototype Bax plays any role in the nTreg cell-specific apoptosis pathways remained unclear (28). To test the proof of principle, we wanted to address two closely related issues: 1) nTreg cells can regulate HLA-A2.1-restricted T cell responses to CML66L; and 2) depleting nTreg cells via increased expression of proapoptotic protein Bax may enhance immune responses to CML66L. Thus, we examined the expression of Bax in nTreg cells in comparison to that of CD4+CD25− T cells. As shown in Fig. 5A, nTreg cells composed 10.3% of peripheral CD4+ T cells, as reported (14). As judged by the higher mean fluorescence intensity (MFI) of Bax signals in nTreg cells, we also found that the expression of Bax in nTreg cells (MFI) = 139.1) was higher than that in CD4+CD25− T cells (MFI = 77.7). Previous studies showed that the 2.6-kb promoter of mouse CD25 targets the reporter gene in T cells with a CD25-specific expression pattern (23). To further examine the role of Bax in nTreg cells, we took use of IL-2-dependent CD25+ CTLL2 T cell line to examine whether overexpression of Bax in nTreg cells triggers apoptosis. A Bax-expressing vector targeting CD25+ T cells was constructed by placing Bax cDNA under the direction of mouse CD25 promoter (Fig. 5B). Of note, using CD25 promoter in this experiment is well justified in the discussion. Transfection of the CD25-Bax vector into CTLL2 T cells resulted in overexpression of c-myc-Bax, as shown by Western blotting with anti-c-myc antibodies (Fig. 5C), and led to higher apoptosis (75%; Fig. 5D) than either that of cells transfected with pCD25 empty vector (30%) or that of untransfected control cells (17%), which corresponded well with the transfection efficiency with this transfection approach (Amaxa) (not shown). Furthermore, transfection of splenocytes with pCD25-Bax also resulted in increased apoptosis of CD4+CD25+ T cells, as shown by the increase in CD25+ and annexin V+ splenocytes from 0.63% (vector transfection control) to 3.52% (CD25-Bax transfection; Fig. 5E). Because only 1.7%–3.5% of splenocytes are CD4+CD25+ T cells (5%–10% of peripheral CD4+ T cells) (14), these results showed that the proapoptotic molecule Bax plays a critical role in the apoptotic pathways of nTreg cells.
Fig. 5. Increased apoptosis of CD4+CD25+ Treg cells via a Bax-dependent mechanism enhances T cell immune responses to CML66L.
A. Higher expression of intracellular Bax in CD4+CD25+ (low and high) T cells than that in CD4+CD25− T cells detected by flow cytometry. In the left figure of the upper panel, CD4+CD25+ T cells (1.46% of total splenic cells) composed of 10.3% of splenic CD4+ T cells (CD4+CD25+ T cells + CD4+CD25-T cells), suggesting a good staining of splenic cells with anti-CD4-PerCP antibodies and anti-CD25-PE antibodies. In the middle figure of the upper panel, CD4+ T cells were divided into CD4+CD25+ T cells (10.3% of CD4+ T cells) and CD4+CD25− T cells (89.7% of CD4+ T cells). In the right figure of the upper panel, a FITC-conjugated antibody isotype control is presented. In the lower panel, as judged by the higher mean fluorescence intensity (MFI), the higher expression of intracellular Bax (MFI=139.1) was observed in CD4+CD25+ T cells than that in CD4+CD25− T cells (MFI=77.7). B. The CD25 promoter-Bax expression vector. A short c-myc expression tag (13 aa) was fused in frame in the N-terminus of Bax. The c-myc-Bax cDNA was subcloned into an expression vector under the direction of a 2.6-kb mouse CD25 promoter. C. The overexpression of c-myc-Bax in IL-2-dependent CD25+ CTLL2 T cells after transfection. The increased expression of c-myc-Bax in transfected CTLL2 T cells was measured with Western blotting using anti-c-myc antibodies (Santa Cruz). D. The increased apoptosis in CTLL2 T cells after transfection of c-myc-Bax cDNA following IL-2 withdrawal. The CTLL2 T cells were transfected with c-myc-Bax or CD25 promoter vector control with the Amaxa nucleofector reagent and instrument, followed by an apoptosis assay with the Annexin V-FITC Apoptosis Detection Kit II. The results of triplicate experiments are presented with the mean and standard deviation. E. The apoptosis rates of nontransfected control cells (the left panel) and CD25+ splenic T cells after transfection using CD25 promoter-c-myc-Bax (the middle panel) or CD25 promoter vector control (the right panel). The apoptosis assays were performed with the Annexin V-FITC Apoptosis Detection Kit II. The representative results presented are from three independent experiments.
Finally, we investigated whether depleting Treg cells by overexpressing Bax could enhance T cell responses to 66Pa. We found that T cell responses, stimulated with irradiated antigen-presenting cells pulsed with 66Pa, were enhanced in CD25-Bax-transfected T cells (23%) but not in T cells transfected with the CD25 promoter empty vector (16.5%), as shown by the percentage of intracellular IFN-γ+CD8+ T cells (Fig. 6A) with statistical significance (p<0.05). The enhanced responses to 66Pa in CD25-Bax-transfected T cells was similar to that seen in CD25-depleted T cells stimulated with 66Pa (25%). The procedure in purification of CD4+CD25+ Treg cells can achieve >90% purity of CD4+CD25+ Treg cells. Since CD62L is a resting T cell marker, CD62L+ was used to gate off potential CD25+ activated T cells (CD62L−) from CD4+CD25+ regulatory T cells (CD62L+)(38). Of note, more than 70% of purified cells were CD4+CD25+CD62L+ (not shown), suggesting that the majority of purified cells were nTreg cells. Therefore, as outlined in Fig. 6C, the results suggest that nTreg cells play an important role in mediation of tolerance to CML66L, as outlined in the upper panel of Fig. 6C. The results also suggest that removal of nTreg cells by inducing apoptosis via a Bax-dependent pathway enhances T cell responses to CML66L, as outlined in the lower panel of Fig. 6C.
DISCUSSION
To avoid the disadvantages of relying on the tumor-bearing patients to identify the T cell epitopes of tumor antigens (15), we used HLA-A2.1 transgenic mice in combination with a novel DNA vaccination approach (16). Our results have shown that CML66L encodes an HLA-A2.1-restricted T cell antigen epitope, 66Pa, which elicits T cell immune responses. 66Pa is naturally presented by CML66L+ human tumor cells in an HLA-A2.1-restricted manner. These findings fit well with our previous results that showed a temporal association of IgG responses to CML66L with CML remission induced by DLI (5), suggesting that CML66L-specific T cells may significantly contribute to anti-tumor responses leading to CML remission (1).
Most tumor antigens identified so far are unmutated self-antigens (9) presumably tolerated. In the development of effective immunotherapy (2), our results have demonstrated that homeostasis of Treg cells is very important in regulation of anti-tumor immune responses (14). Since Treg cells have higher susceptibility to apoptosis (27), our study has further demonstrated for the first time that pro-apoptotic protein Bax is upregulated in unactivated CD4+CD25+ Treg cells and apoptosis of Treg cells is mediated by a Bax-dependent pathway. Using Foxp3 promoter to direct Treg expression of pro-apoptotic protein Bax may also have problems with low efficiency since Foxp3 protein itself promotes CD4+CD25+ Treg cell apoptosis (29). CD25 plays essential roles in CD4+CD25+ Treg development and homeostasis (14). In addition, 96% of CD4+CD25high Treg cells express Foxp3 (30). Thus, using CD25 promoter is justified. Depletion of Treg cells by overexpressed Bax significantly enhances anti-self-leukemia antigen epitope 66Pa T cell responses. These results point out a new direction for enhancement of anti-tumor immunotherapy via regulation of Treg cell homeostasis.
Acknowledgments
We are grateful to Drs. R.F. Wang, R.A. Singh, S. Han, T. Fu, and J.W. Zhang at Baylor College of Medicine; E. Celis at the Mayo Clinic; J. Polo at Chiron Technologies, L. Sherman at the Scripps Research Institute; T. Boon and P.G. Coulie at the Universite de Louvain; P. Reichenbach at the Swiss Institute for Cancer Research; J.P. Zha at the University of Texas Southwestern Medical Center; J.J. Molldrem and D. Galloway at M. D. Anderson Cancer Center for suggestion and assistance. This work was partially supported by NIH grants AI054514; the Leukemia & Lymphoma Society; the Myeloproliferative Disorders Foundation.
References
- 1.Yang F, Yang XF. New concepts in tumor antigens: Their significance in future immunotherapies for tumors. Cell Mol Immunol. 2005;2:331. [PubMed] [Google Scholar]
- 2.Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411:380. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 3.Porter DL, Roth MS, Mcgarigle C, Ferrara JL, Antin JH. Induction of graft-versus-host disease as immunotherapy for relapsed chronic myeloid leukemia. N Engl J Med. 1994;330:100. doi: 10.1056/NEJM199401133300204. [DOI] [PubMed] [Google Scholar]
- 4.Wu CJ, Yang XF, Mclaughlin S, Neuberg D, Canning C, Stein B, Alyea EP, Soiffer RJ, Dranoff G, Ritz J. Detection of a potent humoral response associated with immune-induced remission of chronic myelogenous leukemia. J Clin Invest. 2000;106:705. doi: 10.1172/JCI10196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang XF, Wu CJ, Mclaughlin S, Chillemi A, Wang KS, Canning C, Alyea EP, Kantoff P, Soiffer RJ, Dranoff G, Ritz J. CML66, a broadly immunogenic tumor antigen, elicits a humoral immune response associated with remission of chronic myelogenous leukemia. Proc Natl Acad Sci USA. 2001;98:7492. doi: 10.1073/pnas.131590998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu CJ, Biernacki M, Kutok JL, Rogers S, Chen L, Yang XF, Soiffer RJ, Ritz J. Graft-versus-leukemia target antigens in chronic myelogenous leukemia are expressed on myeloid progenitor cells. Clin Cancer Res. 2005;11:4504. doi: 10.1158/1078-0432.CCR-05-0036. [DOI] [PubMed] [Google Scholar]
- 7.Chen YT, Old LJ. Cancer-testis antigens: Targets for cancer immunotherapy. Cancer J Sci Am. 1999;5:16. [PubMed] [Google Scholar]
- 8.Yan Y, Phan L, Yang F, Talpaz M, Yang Y, Xiong Z, Ng B, Timchenko NA, Wu CJ, Ritz J, Wang H, Yang XF. A novel mechanism of alternative promoter and splicing regulates the epitope generation of tumor antigen CML66-L. J Immunol. 2004;172:651. doi: 10.4049/jimmunol.172.1.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y. SEREX review. Cancer Immunity. 2004 http://www.cancerimmunity.org/SEREX/
- 10.Boon T, Van Der Bruggen P. Human tumor antigens recognized by T lymphocytes. J Exp Med. 1996;183:725. doi: 10.1084/jem.183.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ling M, Wen YJ, Lim SH. Prevalence of antibodies against proteins derived from leukemia cells in patients with chronic myeloid leukemia. Blood. 1998;92:4764. [PubMed] [Google Scholar]
- 12.Schwartz J, Pinilla-Ibarz J, Yuan RR, Scheinberg DA. Novel targeted and immunotherapeutic strategies in chronic myeloid leukemia. Semin Hematol. 2003;40:87. doi: 10.1053/shem.2003.50007. [DOI] [PubMed] [Google Scholar]
- 13.Wang HY, Lee DA, Peng G, Guo Z, Li Y, Kiniwa Y, Shevach EM, Wang RF. Tumor-specific human CD4+ regulatory T cells and their ligands: Implications for immunotherapy. Immunity. 2004;20:107. doi: 10.1016/s1074-7613(03)00359-5. [DOI] [PubMed] [Google Scholar]
- 14.Nomura T, Sakaguchi S. Naturally arising CD25+CD4+ regulatory T cells in tumor immunity. Curr Top Microbiol Immunol. 2005;293:287. doi: 10.1007/3-540-27702-1_13. [DOI] [PubMed] [Google Scholar]
- 15.Lustgarten J, Theobald M, Labadie C, Laface D, Peterson P, Disis ML, Cheever MA, Sherman LA. Identification of HER-2/neu CTL epitopes using double transgenic mice expressing HLA-A2.1 and human CD8. Hum Immunol. 1997;52:109. doi: 10.1016/S0198-8859(96)00292-3. [DOI] [PubMed] [Google Scholar]
- 16.Lachman LB, Rao XM, Kremer RH, Ozpolat B, Kiriakova G, Price JE. DNA vaccination against neu reduces breast cancer incidence and metastasis in mice. Cancer Gene Ther. 2001;8:259. doi: 10.1038/sj.cgt.7700300. [DOI] [PubMed] [Google Scholar]
- 17.Boon T, Van Den Eynde Bj. Cancer vaccines: Cancer antigens. Shared tumor-specific antigens. In: Rosenberg S, editor. Principles and practice of the biologic therapy of cancer. Philadelphia, Baltimore, New York, London, Buenos Aires, Hong Kong, Sydney, Tokyo: Lippincott Williams and Wilkins; 2000. p. 493. [Google Scholar]
- 18.Yang X, Mirkovic D, Zhang S, et al. Processing sites are different in the generation of HLA-A2.1-restricted T cell reactive tumor antigen epitopes and viral epitopes. Int J Immunopathol Pharmacol. 2006;19:853. doi: 10.1177/039463200601900415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molldrem J, Dermime S, Parker K, Jiang YZ, Mavroudis D, Hensel N, Fukushima P, Barrett AJ. Targeted T-cell therapy for human leukemia: Cytotoxic T lymphocytes specific for a peptide derived from proteinase 3 preferentially lyse human myeloid leukemia cells. Blood. 1996;88:2450. [PubMed] [Google Scholar]
- 20.Ye Q, Press B, Kissler S, et al. T cell co-stimulation through CD28 depends on induction of the Bcl-xγ isoform: Analysis of Bcl-xγ-deficient mice. J Exp Med. 2002;196:87. doi: 10.1084/jem.20012084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang HY, Peng G, Guo Z, Shevach EM, Wang RF. Recognition of a new artc1 peptide ligand uniquely expressed in tumor cells by antigen-specific CD4+ regulatory T cells. J Immunol. 2005;174:2661. doi: 10.4049/jimmunol.174.5.2661. [DOI] [PubMed] [Google Scholar]
- 22.Singh RA, Rodgers JR, Barry MA. The role of T cell antagonism and original antigenic sin in genetic immunization. J Immunol. 2002;169:6779. doi: 10.4049/jimmunol.169.12.6779. [DOI] [PubMed] [Google Scholar]
- 23.Soldaini E, Pla M, Beermann F, Espel E, Corthesy P, Barange S, Waanders GA, Macdonald HR, Nabholz M. Mouse interleukin-2 receptor alpha gene expression. Delimitation of cis-acting regulatory elements in transgenic mice and by mapping of DNase-I hypersensitive sites. J Biol Chem. 1995;270:10733. doi: 10.1074/jbc.270.18.10733. [DOI] [PubMed] [Google Scholar]
- 24.Fritze CE, Anderson TR. Epitope tagging: General method for tracking recombinant proteins. Methods Enzymol. 2000;327:3. doi: 10.1016/s0076-6879(00)27263-7. [DOI] [PubMed] [Google Scholar]
- 25.Yang Y, Yang F, Xiong Z, Yan Y, Wang X, Nishino M, Mirkovic D, Nguyen J, Wang H, Yang XF. An N-terminal region of translationally controlled tumor protein is required for its antiapoptotic activity. Oncogene. 2005;24:4778. doi: 10.1038/sj.onc.1208666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, Zheng J, Liu J, Yao J, He Y, Li X, Yu J, Yang J, Liu Z, Huang S. Increased population of CD4(+)CD25(high), regulatory T cells with their higher apoptotic and proliferating status in peripheral blood of acute myeloid leukemia patients. Eur J Haematol. 2005;75:468. doi: 10.1111/j.1600-0609.2005.00537.x. [DOI] [PubMed] [Google Scholar]
- 27.Li L, Godfrey WR, Porter SB, Ge Y, June CH, Blazar BR, Boussiotis VA. CD4+CD25+ regulatory T-cell lines from human cord blood have functional and molecular properties of T-cell anergy. Blood. 2005;106:3068. doi: 10.1182/blood-2005-04-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rathmell JC, Lindsten T, Zong WX, Cinalli RM, Thompson CB. Deficiency in Bak and Bax perturbs thymic selection and lymphoid homeostasis. Nat Immunol. 2002;3:932. doi: 10.1038/ni834. [DOI] [PubMed] [Google Scholar]
- 29.Kasprowicz DJ, Droin N, Soper DM, Ramsdell F, Green DR, Ziegler SF. Dynamic regulation of foxp3 expression controls the balance between CD4(+) T cell activation and cell death. Eur J Immunol. 2005;35:3424. doi: 10.1002/eji.200526339. [DOI] [PubMed] [Google Scholar]
- 30.Roncador G, Brown PJ, Maestre L, Hue S, Martinez-Torrecuadrada JL, Ling KL, Pratap S, Toms C, Fox BC, Cerundolo V, Powrie F, Banham AH. Analysis of Foxp3 protein expression in human CD4(+)CD25(+) regulatory T cells at the single-cell level. Eur J Immunol. 2005;35:1681. doi: 10.1002/eji.200526189. [DOI] [PubMed] [Google Scholar]