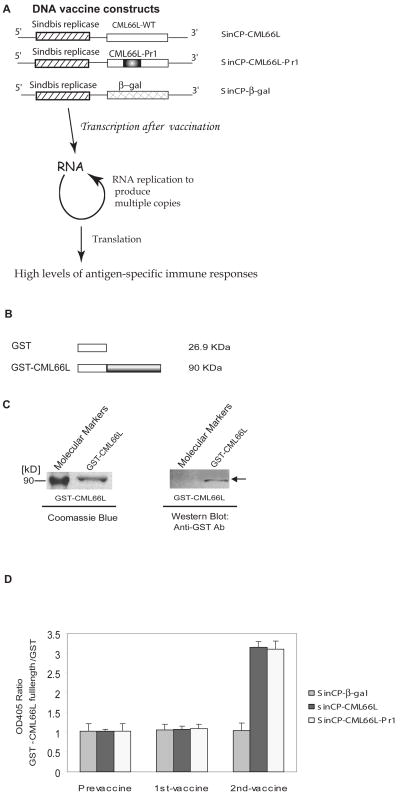
Fig. 2. CML66L elicits IgG antibody responses in HLA-A2.1/Kb transgenic mice after CML66L DNA vaccination.
A. DNA vaccine constructs presented in the upper panel. CML66L cDNA, CML66L-Pr1 cDNA, and β-galactosidase cDNA were subcloned into the pSINCP vector with the Sindbis viral replicon. In the lower panel, the advantage of the high vaccination efficiency of the pSinCP vector in achieving high levels of antigen-specific immune responses is schematically presented. B. Glutathione S-transferase (GST) fusion protein construct of CML66L. The full-length cDNA of CML66L was fused in frame into the C-terminus of GST in the pGEX-3X vector. C. High purity of GST-CML66L fusion protein. The high purity of the purified GST-CML66L was examined with SDS-PAGE followed by staining with Coomassie blue, and the specificity of the purified GST-CML66L was confirmed with Western blotting using anti-GST antibodies, which did recognize the GST-CML66L band (indicated by an arrow) but did not recognize the molecular marker band. D. The specific antibody responses to CML66L and CML66L-Pr1 in vaccinated HLA-A2.1/Kb transgenic mice. The specific antibody responses to CML66L in mouse serum samples were detected by ELISA with purified GST and GST-CML66L as the plate-coating antigens. As described in Materials and Methods, only six of the 583 amino acids in CML66L were mutated to engineer the epitope Pr1 of control antigen proteinase 3 into a position (aa 283-aa 291) on CML66L; thus, the antibody responses to CML66L-Pr1 were examined using GST-CML66L as the ELISA coating antigen, the same as in the examination of anti-CML66L antibody responses with ELISA. The OD405 ratio for each sample was calculated as the OD405 reading of CML66L normalized by the OD405 reading of GST. The ELISA experiment was repeated three times for each sample, and the mean and standard deviation were calculated and presented.