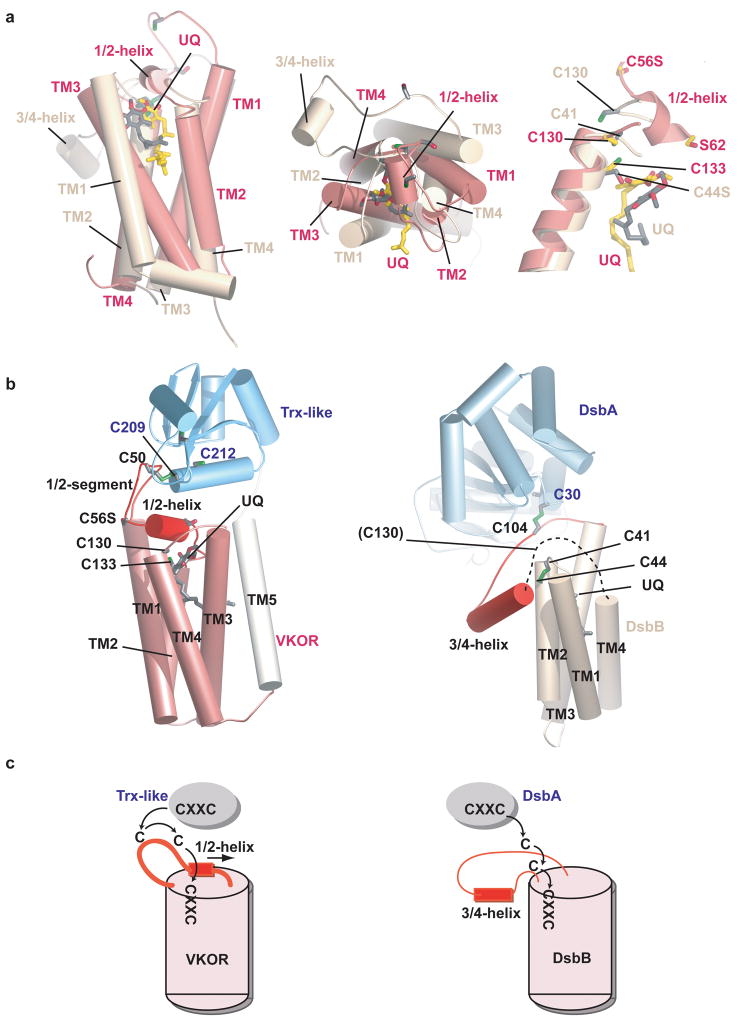
Figure 6. Comparison of VKOR with DsbB.
a, Superposition of the four-helix bundles of VKOR (pink) and DsbB (yellow) 30, based on the helices containing the CXXC motifs. The left two panels show views from the side and periplasm. The right panel shows a close-up view of the active sites. b, Overall architecture of VKOR with its Trx-like redox partner (left panel) and of DsbB with its redox partner DsbA 14. The dotted line in the right panel indicates a segment disordered in the X-ray structure. c, Electron transfer pathways for VKOR and DsbB. Note that for VKOR the loop cysteine cannot access the active site CXXC motif unless the 1/2-helix is displaced. By contrast, in DsbB the 3/4-helix is peripheral and electron transfer can occur without major conformational change; the loop cysteines could not interact if the helix were positioned as in VKOR.