Figure 1. The Protein Kinase Rim15 Directly Phosphorylates Igo1 Both in vitro and in vivo.
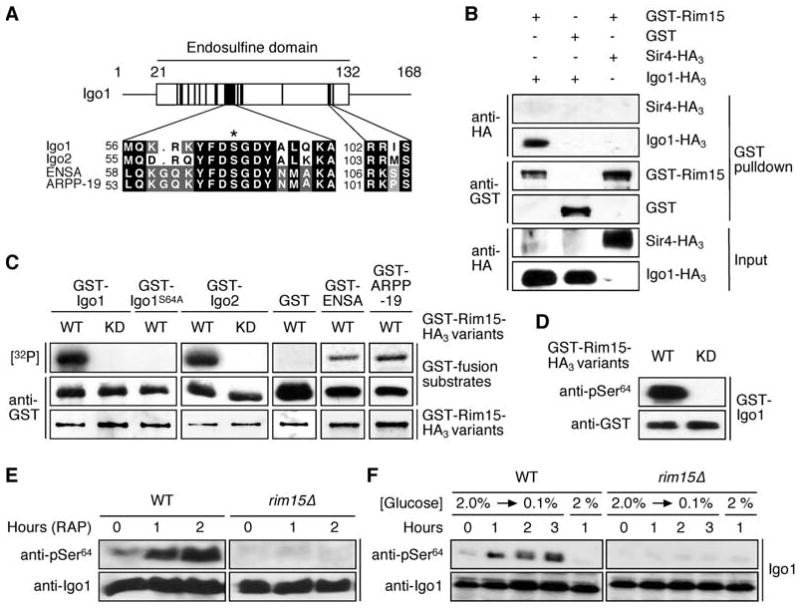
(A) S. cerevisiae Igo1 and Igo2 belong to the conserved family of α-endosulfine proteins. The diagram shows the position of the endosulfine domain within Igo1 with highly conserved subdomains indicated by black boxes. Amino acid sequence alignments of yeast Igo1 and Igo2, and of human ENSA (Swiss-Prot: O43768) and ARPP-19 (Swiss-Prot: P56211) are shown for two of these subdomains. The position of the serine targeted by Rim15 (i.e. Ser64 and Ser63 in Igo1 and Igo2, respectively) is indicated with an asterisk. A conserved PKA consensus phosphorylation site (RR/KXS) is present in the second subdomain.
(B) Rim15 and Igo1 physically interact. GST-Rim15 or GST were pulled down from lysates of wild-type cells coexpressing Igo1-HA3 or, as negative control, Sir4-HA3. Cell lysates (Input) and GST pulldown samples were subjected to SDS-PAGE and immunoblots were probed using anti-HA or anti-GST antibodies.
(C) Rim15 phosphorylates in vitro GST-Igo1, GST-Igo2, GST-ENSA, and GST-ARPP-19, but not GST-Igo1S64A or GST alone. In control experiments, kinase-inactive Rim15KD neither phosphorylated Igo1 nor Igo2. Levels of indicated GST-fusion substrates (purified from E. coli) and of GST-fusion protein kinase variants (GST-Rim15-HA3 [WT] or GST-Rim15KD-HA3 [KD]; purified from yeast) were determined by immunoblot analyses using anti-GST antibodies. [32P] denotes the autoradiograph.
(D) Rim15 specifically phosphorylates Ser64 in Igo1 in vitro. Phosphospecific antibodies directed towards pSer64 in Igo1 recognized bacterially expressed GST-Igo1 following in vitro phosphorylation by active GST-Rim15-HA3 (WT), but not kinase-inactive Rim15KD (KD).
(E, F) In vivo phosphorylation of Ser64 in Igo1 requires the presence of Rim15 and is induced by rapamycin (0.2 μg ml-1) treatment (E) and glucose limitation (F). Levels of Igo1 protein and of Ser64 phosphorylation in Igo1 were determined by immunoblot analyses using polyclonal anti-Igo1 and anti-pSer64 antibodies, respectively.
