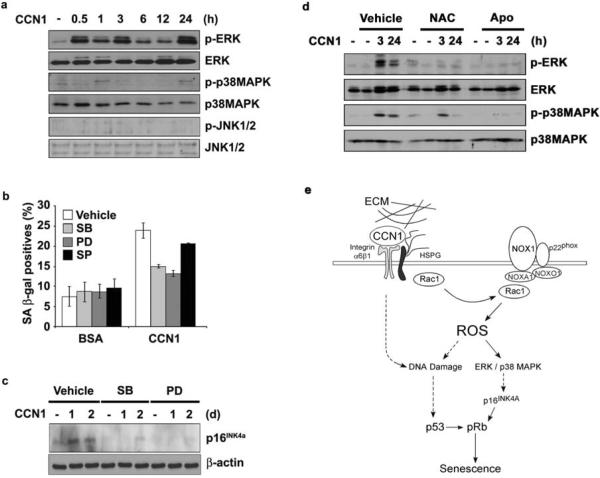
Figure 7. Induction of p16INK4a through ROS-dependent activation of stress kinases.
(a) Lysates of BJ cells treated with CCN1 for indicated times were analyzed for the activation of ERK1/2 (p42/p44), p38 MAPK, and JNK1/2 by immunoblotting using their cognate antibodies and phosho-specific antibodies. (b) Cells were pre-incubated with inhibitors of either p38MAPK (SBS202190; 10 μM), MEK1 (PD98059; 20 μM), or JNK1/2 (SP600125; 25 μM) for 1 h and then treated with CCN1 (2.5 μg/ml). SA-β-gal expression was assayed three days later. Data presented as means ± S.D. (n=5) (c). Cells pre-incubated with inhibitors as above were then treated with CCN1 for 24 or 48 hrs, and p16INK4a was detected by immunoblotting. (d) Activation of both ERK and p38 MAPK by CCN1 was examined in cells pretreated with either NAC (2.5 mM) or apocynin (10 μM) by immunoblotting. (e) A signaling model for CCN1-induced senescence. Upon binding to integrin α6β1 and HSPGs, CCN1 activates the RAC1-NOX1 complex to generate a robust and sustained accumulation of ROS, which triggers the biphasic hyperactivation of ERK and p38 MAPK, leading to p16INK4a induction. CCN1 also induces a DDR and activates p53, in part through a ROS-dependent mechanism. Both p53 and p16INK4a contribute to CCN1-induced senescence.