Abstract
To clarify the role of insulin and growth hormone (HGH) in regulating substrate production for body fuel during prolonged starvation, 6 normal subjects and 10 HGH-deficient dwarfs were fasted for 6 days. Four of these dwarfs received HGH during the fast.
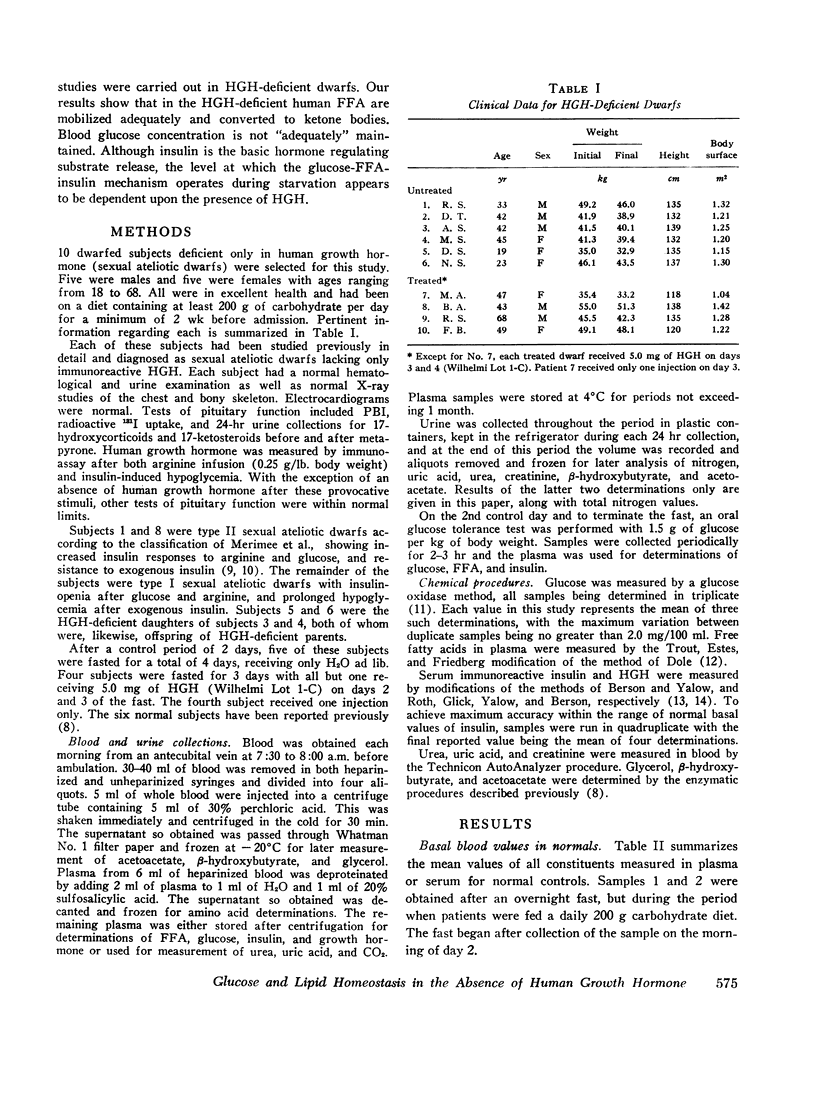
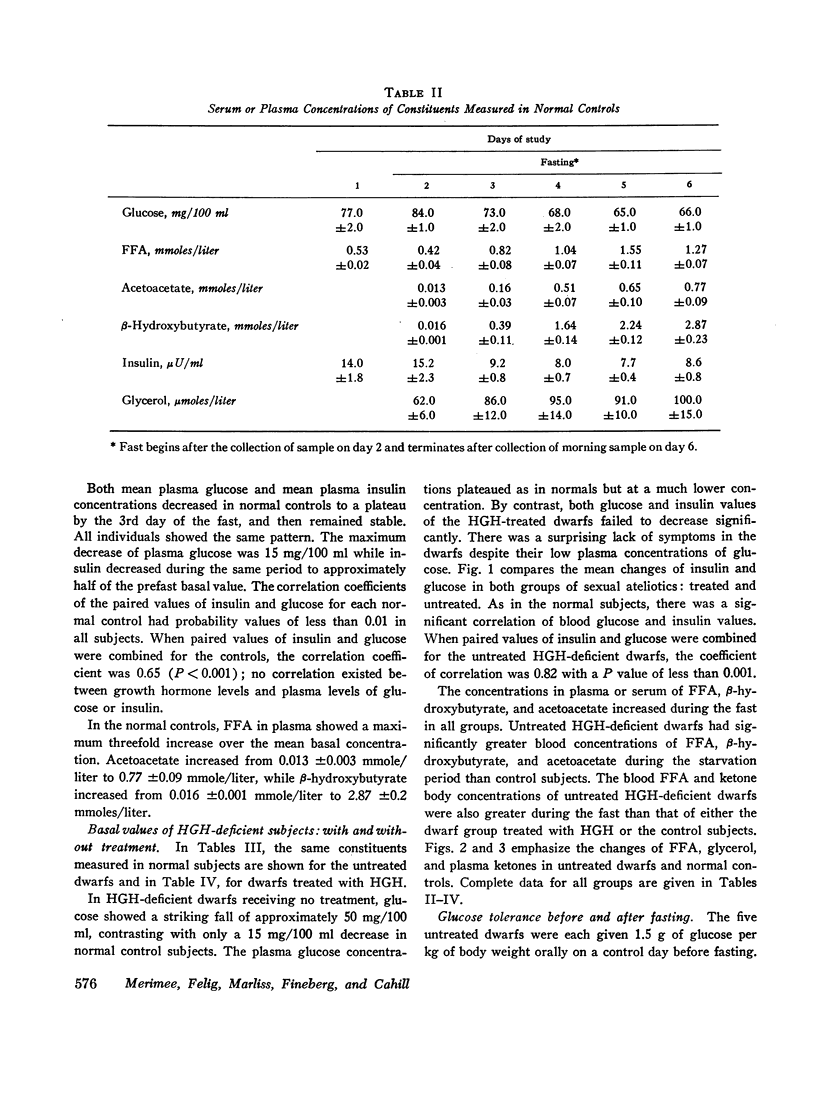
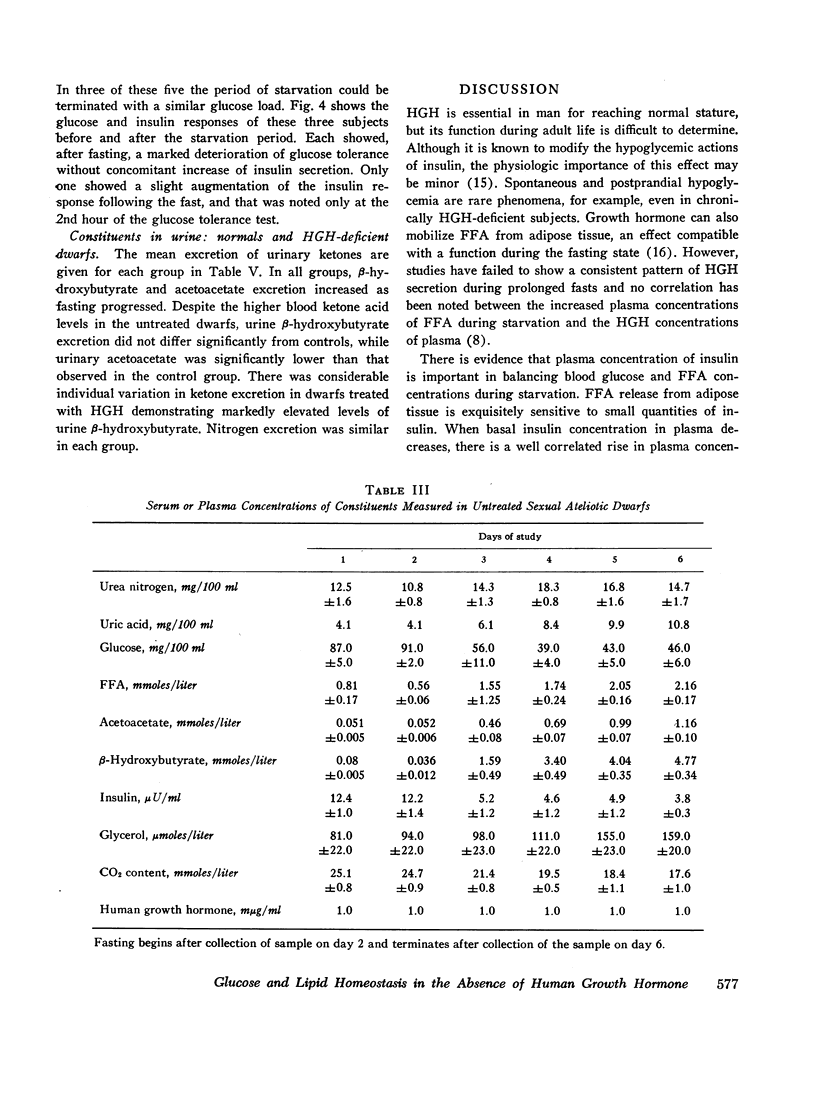
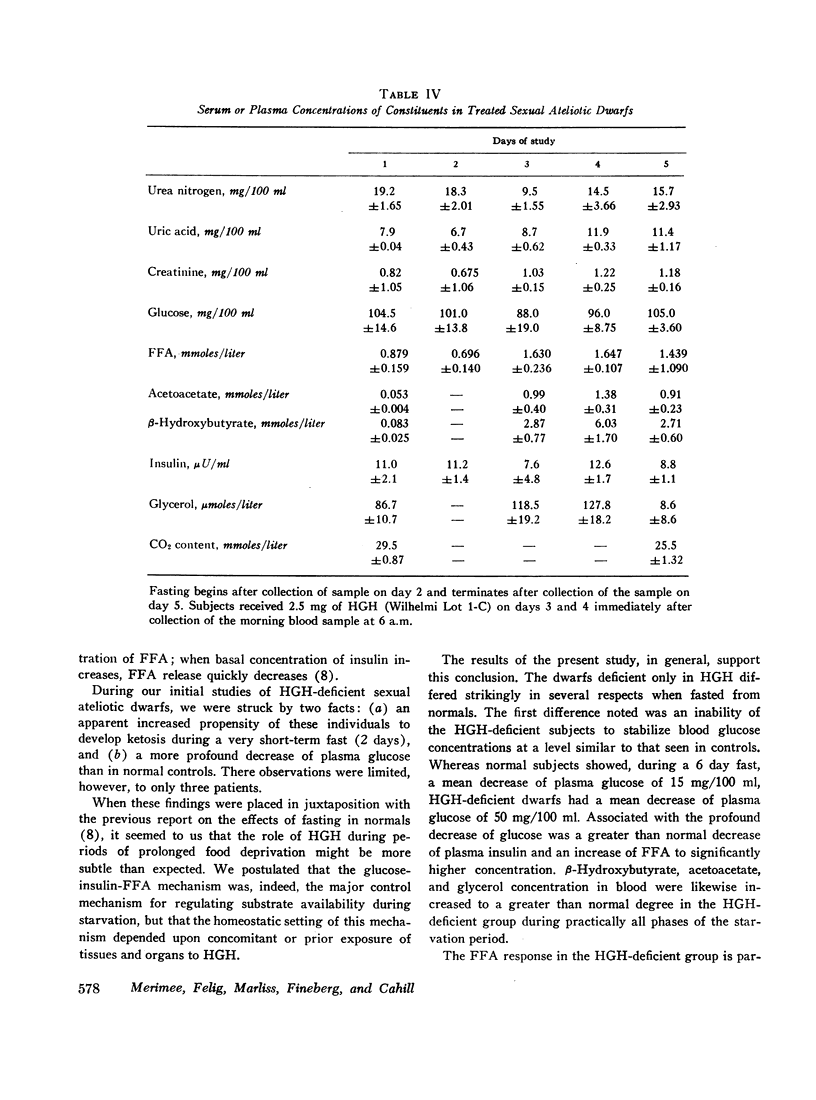
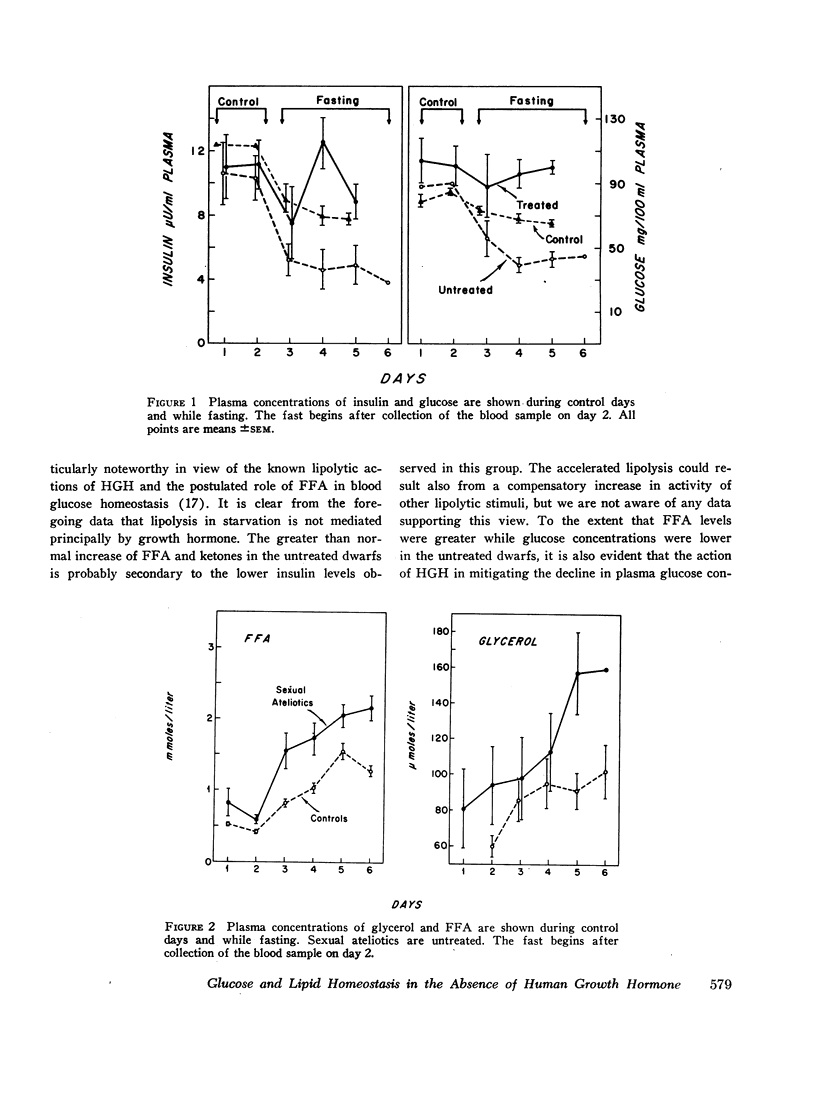
Blood glucose concentration decreased a mean 15 mg/100 ml in both controls and HGH-treated dwarfs, but decreased 50 mg/100 ml in untreated dwarfs. The final level at which the blood glucose stabilized was significantly higher in the former two groups (65 ±1.0 mg/100 ml and 88 ±19 mg/100 ml, respectively, versus 39.0 ±4.0 mg/100 ml in the untreated dwarfs). The decline in plasma insulin concentration showed a comparable pattern, decreasing from a similar basal level to 7.7 ±0.4 μU/ml in controls, 8.8 ±1.1 μU/ml in dwarfs treated with HGH, and to a significantly lower level of 3.8 ±1.1 μU/ml in untreated dwarfs. When glucose concentrations were plotted against paired insulin values, the correlation in both dwarfs and normals was significant. In normals, no correlation existed at any time between plasma HGH levels and plasma concentration of either glucose or free fatty acid.
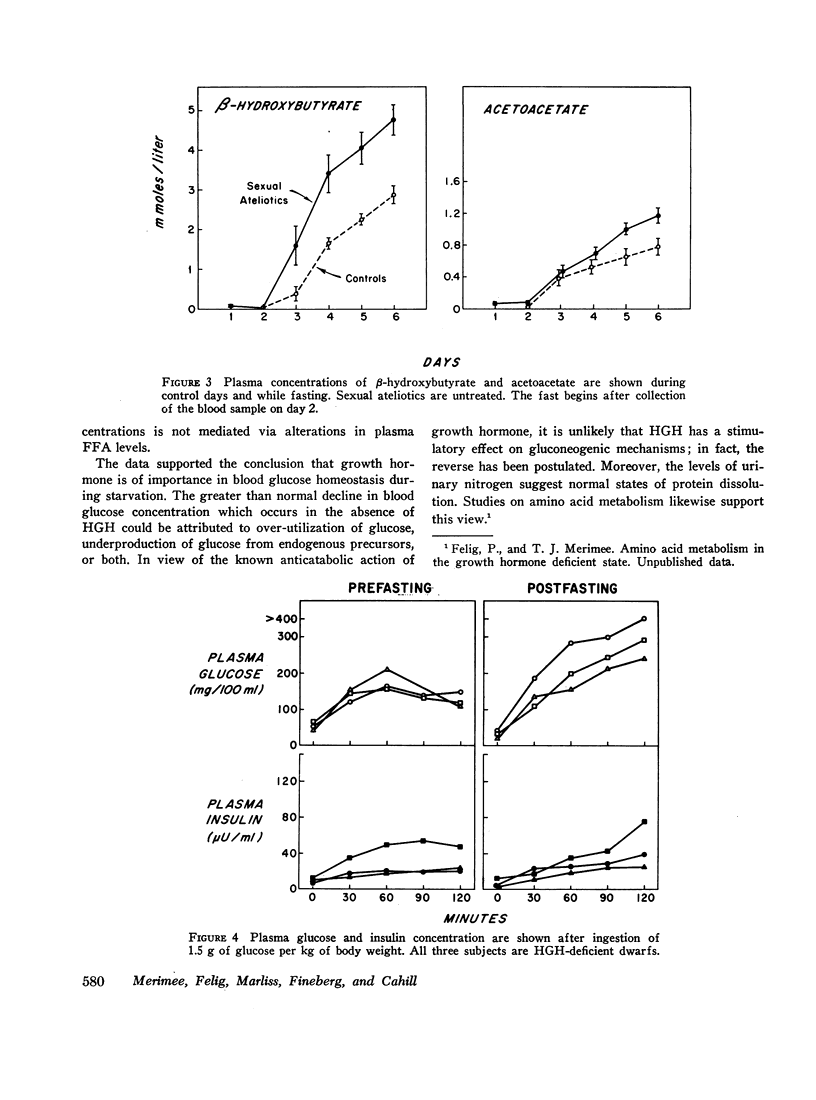
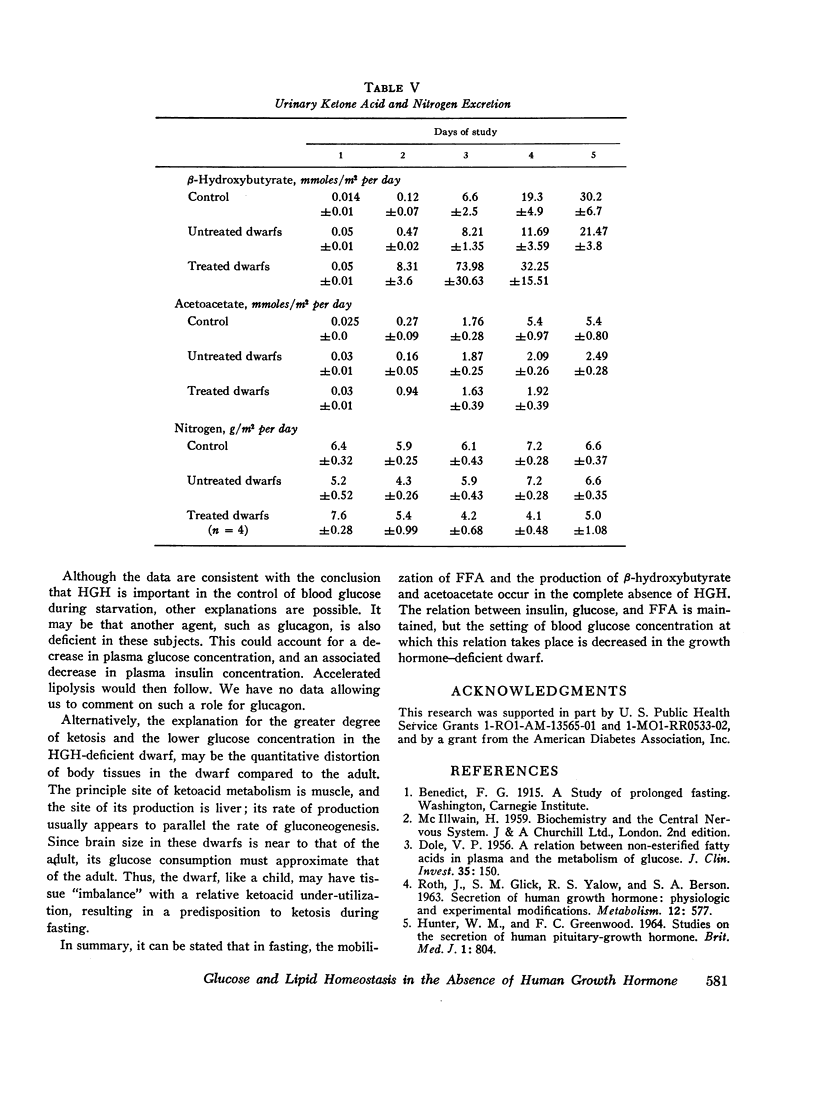
Free fatty acid, β-hydroxybutyrate, and acetoacetate increased respectively in normals to peak concentrations in plasma of 1.55 ±0.11, 2.87 ±0.23, and 0.77 ±0.09 mmoles/liter. Untreated dwarfs had significantly greater values of all three (mean maximal concentration: FFA = 2.16 ±0.17 mmoles/liter, β-hydroxybutyrate = 4.11 ±0.34 mmoles/liter, and acetoacetate = 1.16 ±0.10 mmoles/liter). Values returned toward normal in HGH-treated dwarfs. The cahnges in plasma concentrations of β-hydroxybutyrate and acetoacetate were not due to changes in renal excretion.
In starvation, the relation between insulin on the one hand and glucose and free fatty acid on the other hand is maintained in the absence of HGH. However, the setting of blood glucose concentration at which this relation takes place is decreased in the absence of HGH. This results in a lower than normal insulin level and, consequently, in a higher than normal free fatty acid concentration.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BECK J. C., McGARRY E. E., DYRENFURTH I., VENNING E. H. The metabolic effects of human and monkey growth hormone in man. Ann Intern Med. 1958 Nov;49(5):1090–1105. doi: 10.7326/0003-4819-49-5-1090. [DOI] [PubMed] [Google Scholar]
- Cahill G. F., Jr, Herrera M. G., Morgan A. P., Soeldner J. S., Steinke J., Levy P. L., Reichard G. A., Jr, Kipnis D. M. Hormone-fuel interrelationships during fasting. J Clin Invest. 1966 Nov;45(11):1751–1769. doi: 10.1172/JCI105481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOLE V. P. A relation between non-esterified fatty acids in plasma and the metabolism of glucose. J Clin Invest. 1956 Feb;35(2):150–154. doi: 10.1172/JCI103259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLICK S. M., ROTH J., YALOW R. S., BERSON S. A. IMMUNOASSAY OF HUMAN GROWTH HORMONE IN PLASMA. Nature. 1963 Aug 24;199:784–787. doi: 10.1038/199784a0. [DOI] [PubMed] [Google Scholar]
- GLICK S. M., ROTH J., YALOW R. S., BERSON S. A. THE REGULATION OF GROWTH HORMONE SECRETION. Recent Prog Horm Res. 1965;21:241–283. [PubMed] [Google Scholar]
- HUGGETT A. S., NIXON D. A. Use of glucose oxidase, peroxidase, and O-dianisidine in determination of blood and urinary glucose. Lancet. 1957 Aug 24;273(6991):368–370. doi: 10.1016/s0140-6736(57)92595-3. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. STUDIES ON THE SECRETION OF HUMAN-PITUITARY-GROWTH HORMONE. Br Med J. 1964 Mar 28;1(5386):804–807. doi: 10.1136/bmj.1.5386.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera M. G., Kamm D., Ruderman N., Cahill Non-hormonal factors in the control of gluconeogenesis. Adv Enzyme Regul. 1966;4:225–235. doi: 10.1016/0065-2571(66)90017-3. [DOI] [PubMed] [Google Scholar]
- Merimee T. J., Hall J. D., Rimoin D. L., McKusick V. A. A metabolic and hormonal basis for classifying ateliotic dwarfs. Lancet. 1969 May 10;1(7602):963–965. doi: 10.1016/s0140-6736(69)91861-3. [DOI] [PubMed] [Google Scholar]
- Merimee T. J., Rabinowitz D., Rimoin D. L., McKusick V. A. Isolated human growth hormone deficiency. 3. Insulin secretion in sexual ateliotic dwarfism. Metabolism. 1968 Nov;17(11):1005–1011. doi: 10.1016/0026-0495(68)90006-1. [DOI] [PubMed] [Google Scholar]
- RABEN M. S., HOLLENBERG C. H. Effect of growth hormone on plasma fatty acids. J Clin Invest. 1959 Mar;38(3):484–488. doi: 10.1172/JCI103824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTH J., GLICK S. M., YALOW R. S., BERSON S. A. Secretion of human growth hormone: physiologic and experimental modification. Metabolism. 1963 Jul;12:577–579. [PubMed] [Google Scholar]
- TROUT D. L., ESTES E. H., Jr, FRIEDBERG S. J. Titration of free fatty acids of plasma: a study of current methods and a new modification. J Lipid Res. 1960 Apr;1:199–202. [PubMed] [Google Scholar]
- YALOW R. S., BERSON S. A. Immunoassay of endogenous plasma insulin in man. J Clin Invest. 1960 Jul;39:1157–1175. doi: 10.1172/JCI104130. [DOI] [PMC free article] [PubMed] [Google Scholar]


