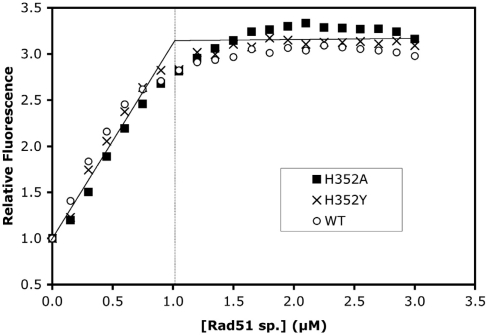
Figure 2.
ssDNA-binding site size determination for wild-type and mutant Rad51 proteins. Etheno-modified M13mp19 ssDNA (εDNA) was titrated with Rad51 wild-type (open circles), H352A (filled squares), or H352Y (crosses) under low-salt conditions while monitoring the enhancement of εDNA fluorescence as described under ‘Materials and Methods’ section. Solutions contained 3 µM εDNA and 2 mM ATP in buffer consisting of 30 mM Tris–acetate (pH 7.5), 10 mM magnesium acetate and 0.1 mM DTT. The apparent binding site sizes of proteins on this single-stranded lattice are estimated from the titration endpoints. The average endpoint is indicated in this figure by the intersection of the solid asymptotic lines, with the vertical line showing the protein concentration at the average endpoint, corresponding to one Rad51 protomer per three εDNA nucleotide residues.