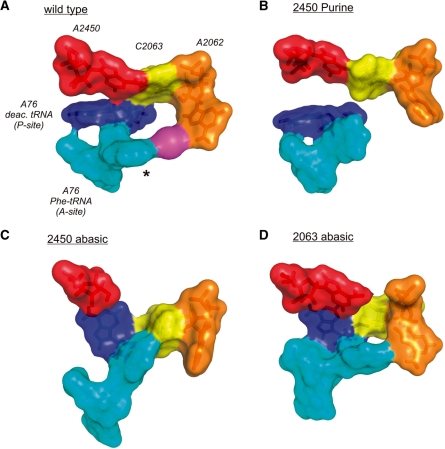
Figure 6.
Effects of modulating the A2450–C2063 pair on the PTC architecture. (A) In the surface representation of the structure obtained at the end of the MD simulation of the wt PTC, a continuous ‘interaction ring’ is formed involving the A2450–C2063 pair, A2062, a constrained monovalent ion (magenta), the phenylalanine side chain (asterisk) of A-site bound Phe-tRNAPhe and A76 of deacylated tRNA in the P-site. (B) Removal of the adenine N6-amino group by introducing purine at residue 2450 resulted in the loss of base pairing with C2063 and a complete disconnection of A2062 and the A-site tRNA. (C) Removal of the entire nucleobase at 2450 severely affected the PTC architecture also resulting in the loss of interactions of A2062 with the aa-tRNA in the A-site. Instead the amino acid side chain of Phe-tRNAPhe seems to interact with the nucleobase at C2063. (D) Removal of the base at 2063 showed less dramatic effects since A2062 was still able to reach the aa-tRNA in the A-site. However, the A2450–C2063 base pair is destroyed as well as A76 of P-tRNA is positioned significantly different in respect to A2450 as compared to the wt situation in (A).