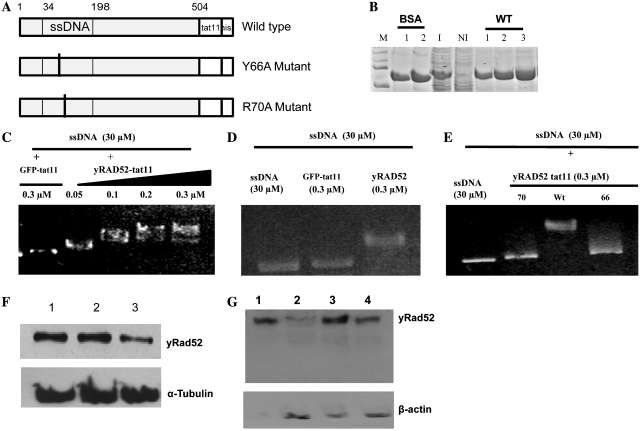
Figure 1.
Purification, ssDNA binding activity and delivery of yRad52tat11. (A) Maps of the yRad52tat11 wild-type fusion protein, the Y66A and the R70A mutant, that for simplicity only show the N-terminal DNA binding domain; the numbers represent amino acid residues in the wild-type yeast molecule; the ssDNA binding domain is shown. Both mutations are located in this domain; the tat11 and the his-tag are located at the C-terminus. (B) Coomassie staining of purified wild-type-yRad52-tat11 protein (about 52 kDa); M-lane, markers; BSA-lanes 1 and 2, 200 and 400 ng of bovine serum albumin; I-lane, 5 µg of total protein extract from IPTG-induced bacteria; NI-lane, 5 µg of total protein extract from not induced bacteria; WT-lanes 1, 2 and 3, wild-type yRad52tat11 purified protein elutions; the yield was 80, 70 and 50 ng/μl for three different elutions. (C) In vitro mobility shift assay with ssDNA (30 µM as nucleotides) and increasing concentrations (0.05, 0.1, 0.2, 0.3 µM) of wt yRad52tat11 protein or GFPtat11 (0.3 µM) as control; the reaction mixtures were loaded in a 0.9% agarose gel, electropheresed and stained with ethidium bromide. (D) In vitro mobility shift assay with ssDNA (30 µM as nucleotides) alone, in presence of 0.3 µM concentration of wild-type yRad52 or GFPtat11. (E) In vitro mobility shift assay with ssDNA (30 µM as nucleotides) alone or in presence of 0.3 µM concentration of wild-type yRad52tat11, R70A or Y66A mutated protein, as indicated. (F) Western blot analysis of total cell extracts; 6 × 105 HeLaG1 cells were incubated with 20 µg/ml wild-type-yRad52tat11 or mutant proteins for 24 h; cell lysis and total cell extracts were carried out as reported in ‘Materials and Methods’ section; 10 µg of total proteins were loaded in each well; the blot was hybridized with anti-penta-6-histidine antibody; lane 1, extract from wild-type yRad52tat11-exposed cells; lane 2, extract from R70A treated cells; lane 3, extract from Y66A treated cells; tubulin was used as loading control. (G) Western blot analysis of nuclear extracts from 3 × 106 total cells treated with 20 µg/ml of wild type or mutated yRad52tat11 protein for 24 h; aliquots corresponding to 10 µg of nuclear protein extract were loaded and electrophoresed; lane 1, 1 µg of purified yRad52tat11protein; lane 2, nuclear extract from Y66A mutant-treated cells; lane3, nuclear extract from wild-type yRad52tat11-treated cells; lane 4, nuclear extract from R70A mutant-treated cells.