Abstract
Despite the ability of cancer vaccines to induce tumor-specific T-cells in the blood of patients with cancer, and early, promising data indicating their ability to delay cancer progression, their ability to induce cancer regression remains low. The use of ex vivo-generated dendritic cells (DCs) in such vaccines can help to sidestep the cancer-associated dysfunction of endogenous DCs and to deliver the key instructive signals needed for effective antitumor responses. Effective ways of loading DCs with tumor-related antigens, while retaining the high costimulatory function required for T-cell expansion (ie, effective delivery of 'signal one' and 'signal two'), have been previously identified. More recently, different DC populations have been found to deliver a specialized third signal, able to regulate the acquisition of desirable T-cell effector functions, as well as an additional fourth signal that regulates the homing properties of T-cells. Moreover, ex vivo instruction of DCs can be used to preferentially activate CTLs, T-helper 1 and NK cells, while limiting the undesirable activation of regulatory T-cells. These developments can result in the induction of T-cells with desirable effector functions and tumor-relevant homing properties, even in the absence of proinflammatory signals (typically present in recall infections, but not in advanced cancer), thus helping to bridge the gap between the effectiveness of therapeutic and preventive cancer vaccines.
Keywords: Cancer, chemokine, CTL, cytokine, dendritic cell, IL-12, immunotherapy, NK cell, Th1, vaccine
Introduction
Despite advances in cancer prevention and therapy and the recently noted decrease in cancer-related deaths in the annual report from the American Cancer Society, CDC, NCI and the North American Association of Central Cancer Registries, cancer remains a leading cause of mortality [1,2], with substantial numbers of patients lacking effective treatment and even larger numbers lacking definitive cure. The combined use of surgery, radiotherapy and chemotherapy is often highly successful in eliminating the major tumor mass, but is less effective in eliminating residual cancer cells and in preventing disease recurrence. This particular disadvantage of the currently available treatments has provided the rationale for utilizing the immune system (specialized in eliminating 'rare events' in our bodies, such as invading bacteria or host cells hijacked by viruses) in a therapeutic context to identify and destroy cancer cells.
Therapeutic cancer vaccines attempt to instruct the patients' own immune system to kill cancer cells. They have the unique advantages of low toxicity and of being able to target multiple target molecules, even newly arising antigens on rapidly-mutating tumor cells. Following the initial demonstration by William Coley that the immune system can be mobilized to fight established cancer, large research efforts have helped to understand the basic principles governing immune recognition and elimination of tumor cells [3].
Recent observations that cancer vaccines, particularly dendritic cell (DC)-based vaccines, are able to induce 'epitope spreading', that is, to extend the antigenic spectrum of responses beyond those observed with the original vaccine [4,5], further support the ability of vaccines to target heterogeneous tumor-cell populations, despite the adaptability and antigenic mimicry associated with such cells.
However, despite the increasingly high immunological effectiveness of new cancer vaccines and indications of their ability to delay cancer progression [6], currently available therapeutic vaccines against cancer are still not as effective as preventive vaccination is against infective agents [6–14]. In particular, while current cancer vaccines exhibited early promise in inducing disease stabilization and prolonging patients' survival [12,15–17], they have remained poorly effective in inducing regression of bulky tumors [11].
Therapeutic vaccines: Old paradigms and new challenges
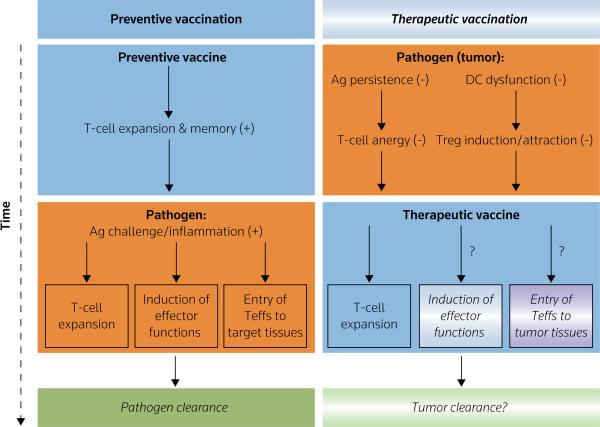
Compared with preventive vaccines, which aim to induce the expansion of pathogen-specific T-cells and to establish immune memory, therapeutic vaccines need to successfully overcome several challenges that are unique to the setting of established cancer (Figure 1). In contrast to responses to tissue-invading microorganisms mediated by immunological memory, vaccination-induced T-cells in patients with cancer are not exposed to proinflammatory danger signals from infected tissues and innate immune cells, which are known to facilitate the development of T-cell effector functions and their attraction to the sites of pathogen entry [18–20]. This introduces more stringent requirements for therapeutic vaccines, which in addition to driving the expansion of cancer-specific T-cells, must also directly induce the acquisition of T-cell effector functions and tumor-relevant homing potential.
Figure 1. Therapeutic versus protective vaccines.
In contrast to recall responses to tissue-invading microorganisms, T-cells in therapeutically vaccinated patients with cancer are not exposed to proinflammatory alarm signals (typically present in infected tissues), thus introducing the need for therapeutic vaccines, or additional components of immunotherapy, to induce the acquisition of tumoricidal effector functions and tumor-relevant homing potential. Additional problems in patients with cancer, who are persistently exposed to tumor-related antigens and immunosuppressive factors, include the dysfunction of endogenous dendritic cells (DCs) and other types of APCs that exhibit reduced ability to stimulate effector T-cells [30–43], as well as the hyperactivation of regulatory T-cells (Tregs) able to suppress active immunity [24–27]. Therefore, the effectiveness of therapeutic vaccines in such individuals is likely to require the presence of a fully mature DC population, resistant to suppression and able to either avoid or resist the interaction with immunosuppressive Tregs. Orange: pathogen-dependent events; blue: vaccination-dependent events; gradient: incomplete or only partial effectiveness, (+): positive/desirable effect; (−) suppressive/undesirable effect.
Ag antigen, Teffs effector T-cells
The immune suppression associated with advanced cancer and the dysfunction of endogenous DCs and other APCs [21–23], whose function is to stimulate effector T-cells, as well as the hyperactivation of regulatory T-cells (Tregs) [24–27], which are able to suppress active immunity, are additional factors that need to be taken into consideration in therapeutic vaccine development. Endogenous DCs in cancer-bearing patients are a target of tumor-associated suppressive factors, resulting in their aberrant function and thus, the impaired induction of effector activity in tumor-specific lymphocytes [28,29]. Mediators of tumor-induced DC dysfunction include IL-10, TGFβ, VEGF, IL-6 and prostanoids, such as PGE2, generally overproduced in cancer [21–34]. DCs developing in the presence of such factors fail to mature, and are thus unable to express sufficient levels of costimulatory molecules required for T-cell activation, or to produce the cytokines required to support the survival and effector functions of tumor-specific T-cells [35–38]. Dysfunction of endogenous DCs has been observed in patients with melanoma, ovarian, breast, renal, prostate, lung and head and neck cancer [36,39–43]. The absence of adequate costimulation and cytokine secretion by DCs leads to naïve, memory and effector T-cell anergy, thus favoring tumor evasion [30–34]. In addition to the dysfunction of endogenous DCs, patients with advanced cancer often show expansion and hyperactivation of Tregs [24–27]. Tregs limit the effectiveness of cancer vaccines [26,44], and they have been found to be preferentially expanded in the presence of at least some of the currently used cancer vaccines [27].
The above observations raise concerns as to whether the traditional vaccine paradigms, developed based on the experience with current protective vaccines, are relevant to, or sufficient for the development of therapeutic vaccines against cancer. Even though, similar to preventive vaccines, therapeutic vaccines need to be effective in inducing the expansion of cancer-specific T-cells, as well as their subsequent development into memory cells, they must also be effective in overcoming tumor-associated immune dysfunction/suppression and may need to 'adopt' the role of proinflammatory cytokines and chemokines (typically present in infected tissues) in inducing tumoricidal effector functions and tumor homing.
Ex vivo-generated dendritic cells in cancer treatment: Dendritic cell-based cancer vaccines
The dysfunction observed in endogenous DCs in patients with cancer suggested the use of ex vivo-generated DCs as carriers of cancer vaccines [45]. DCs, originally identified by Ralph Steinman in 1975 [46–49], are APCs uniquely specialized in inducing primary immune responses, supporting the survival and effector functions in previously primed T-cells and mediating overall communication within the immune system [50,51]. As, in contrast to DCs that develop in the context of tumor-related suppressive factors, fully mature DCs acquire at least partial resistance to such mediators [52–54], ex vivo-generated DCs have been extensively used as a therapeutic tool.
Selecting the right tools for cancer vaccines: Dendritic cells as carriers of signals 1, 2 and 3
DCs provide T-cells with an antigen-specific 'signal 1' and a costimulatory 'signal 2' [55–57], both of which are required for the activation and expansion of pathogen-specific T-cells. DCs also provide an additional third signal (signal 3), which polarizes the development of immune responses toward T-helper-1 cell (Th1) or Th2 responses (type-1 or type-2 immunity, which is desirable and undesirable in cancer, respectively) [51], thus leading to differential activation of particular effector mechanisms, as well as different capabilities in inducing cancer rejection [51,55–64]. In addition to their role as initiators of antigen-specific Th responses, DCs also activate and support the tumoricidal functions of NK cells [65,66]. Also, effective induction of antitumor CTL responses requires mature DCs expressing high levels of costimulatory molecules and that are able to migrate in response to CCL19 (chemokine [C-C motif] ligand 19) or CCL21, the lymph node-produced CCR7 (chemokine [C-C motif] receptor 7) ligands [67–70]. In addition, high IL-12p70 secretion dramatically enhances the ability of DCs to induce tumor-specific Th1 and CTL activation, and to promote tumor rejection in therapeutic mouse models [71–81].
Unfortunately, obtaining DCs with high immunostimulatory function, high migratory activity and high capacity to produce IL-12p70 proved to be difficult. First-generation DC-based vaccines utilized relatively immature or partially mature DCs, which were immunogenic and able to promote stabilization or regression of cancer in a proportion of patients [82,83], but were suboptimal with regard to their lymph-node homing ability and T-cell-stimulating potential [67,68]. Second-generation DC-based vaccines used cells which were fully mature in terms of costimulatory and homing function (matured in the presence of an IL-1β/TNFα/IL-6/PGE2-containing cytokine cocktail [84]), but exhibited a reduced ability to produce bioactive IL-12p70, a phenomenon referred to as 'DC exhaustion' [52,85,86]. Thus, although second-generation DC-based vaccines were clearly superior to the immature/partially mature DCs used in the first-generation vaccines with respect to their immunogenic capacity [67,68] and migratory responses to lymph node-associated chemokines [70,87,88], the combination of these two properties with the production of IL-12p70 was difficult to attain.
Based on the above observations, the feasibility of inducing 'non-exhausted' mature DCs was examined as a means of boosting the clinical efficacy of cancer vaccines [53,86,89–95]. More specifically, immature DCs were exposed to Th1- and Th2-associated IFNs and T-cell receptor (TLR) ligands, or, alternatively, to properly-activated NK cells or CD8+ memory T-cells. The resulting 'type-1 polarized' DCs (DC1s) exhibited a dramatically enhanced capacity to induce long-lived tumor-specific T-cells with strongly pronounced antitumor effector functions in both human in vitro and mouse in vivo models, as well as the ability to enhance tumoricidal functions of resting NK cells. The original observations from the Pawel Kalinski research group [53,86], in combination with data from the laboratories of Brian Czerniecki in Philadelphia, PA, USA [93] and Marieke van Ham in Amsterdam [96], demonstrated that culturing DCs with a combination of IFNγ and LPS (including monophosphoryl lipid A, the clinical-grade form of LPS) or in the presence of the maturation-inducing cytokines TNFα and IL-1β was able to overcome the maturation-associated 'exhaustion' of the cells, yielding stable DC1s able to produce highly elevated levels of IL-12p70 upon interaction with CD40L-expressing (ie, activated) CD4+ Th cells and to induce more potent Th1 and CTL responses (as assessed by increased tumor-cell recognition) [53,93]. DC1s with similar properties can be effectively induced by 'two-signal-activated' autologous NK cells or CD8+ memory T-cells ([91,92,95] and [Kalinski P: unpublished data]). Further addition of IFNα and polyinosinic:polycytidylic acid (poly I:C) to the DC maturation cocktail enhanced the ability of maturing DCs to acquire CCR7 expression [86], and instructed the cells to preferentially interact with naïve, memory and effector T-cells, rather than with Tregs [34] (see below). These results suggested that polarized DCs may be able to avoid the undesirable expansion of Tregs observed with previously used cancer vaccines [27,97–100].
In accordance with the ability of polarized DCs to induce qualitatively improved immune responses, 'α-type-1-polarized' DCs (αDC1s; cultured in IFNα/poly I:C/TNFα/IL-1β/IFNγ) induced higher numbers of long-lived, functional, melanoma-specific CTLs (on average 20-fold higher) following a single round of in vitro sensitization [86], when directly compared with standard (s)DCs, matured in the presence of IL-1β/TNFα/IL-6/PGE2 [84], a maturation protocol frequently used in second-generation DC-based vaccines. Data from melanoma [86], chronic lymphocytic leukemia [101], and several other cancers, uniformly demonstrated the feasibility of generating DC1s from patients with advanced cancer and loading them with peptide antigens [86] or apoptotic tumor cells [101] to induce tumor-specific CTLs.
Based on the promising results from the first clinical trials involving therapeutic vaccines using partially mature first-generation DCs in follicular lymphoma and melanoma [82,83] in the mid-1990s, DCs have since been used to treat patients with several different malignancies. Even though the rates of clinical responses (as measured by RESIST [response evaluation criteria in solid tumors] or WHO criteria) rarely exceed 10 to 15% [11,15–17,45,102], recent data from phase III clinical trials of a first-generation DC vaccine against prostate cancer (Sipuleucel-T/Provenge, Dendreon Corp) demonstrated that it prolonged the overall survival of vaccinated patients [16,103,104], thus raising the question of whether clinical responses (measured by RESIST criteria that were developed to monitor the direct cytotoxic effects of chemotherapeutic agents) can accurately predict the long-term advantage of cancer vaccines [6,13].
Intriguingly, while second-generation DC vaccines, matured in the IL-1β/TNFα/IL-6/PGE2-containing cytokine cocktail [84], are clearly superior to immature DCs with respect to their immunogenic capacity [67,68] and migratory responses to lymph node-associated chemokines [70,87,88], as previously discussed, a recent phase III clinical trial of autologous, peptide-pulsed DCs in advanced melanoma demonstrated that their effectiveness (< 10%) was not superior to dacarbazine treatment [105]. The reason for this disappointing result remains unclear; however, the undesirable negative impact of PGE2 on the production of IL-12 [34,53,54,106], a cytokine which has numerous activities central to the induction and survival of type-1 immune cells [61], and the susbequent activation of Tregs [27,98] are possible culprits in this respect.
The clinical efficacy of third-generation DC-based vaccines using DC1s is currently being evaluated at the University of Pittsburgh Cancer Institute, in clinical trials in cutaneous T-cell lymphoma, glioma, melanoma and colorectal cancer (ClinicalTrials.gov identifiers: NCT00099593, NCT00766753, NCT00390338 and NCT00558051, respectively), with early clinical data expected to be available in 2010, whereas a trial in prostate cancer was expected to begin shortly after the time of publication [G Chatta, personal communication]. While the IFN-induced type-1-polarization of DCs, used to avoid the 'exhaustion' of mature DCs [53,86,93,96], offers a clinically applicable way of enhancing the desirable features of DCs used as cancer vaccines, the effectiveness of this approach needs to be compared (or possibly combined) with other ways of enhancing the desirable properties of DCs. Such potential alternatives and combinations may include the use of IL-15 (rather than IL-4) to promote early stages of DC development [107], the use of B7-DC cross-linking [108], inhibition of p38MAPK [109,110] as alternative ways of enhancing T-cell activation, or inclusion of multiple TLR ligands that can have a synergistic effect in the induction of bioactive IL-12p70 and prime DCs for high IL-12 production during the interaction with T-cells [111–114], thus generating DCs able to produce high levels of proinflammatory cytokines, as well as having other desirable features, as discussed below.
DCs as mediators of signal 4: Induction of tumor-specific T-cells with tumor-relevant homing potential
Differences in the homing properties of different T-cell subsets have been known for some time [115–121], but it was only in the last 6 to 7 years that it became apparent that DCs play an important role in the regulation of T-cell homing characteristics [122–126]. DCs use vitamins A and D to induce the T-cell chemokine receptors, CCR9 [127] and CCR10 [128], thus allowing T-cells to preferentially migrate to the gut or the skin. In addition, DCs from Peyers' patches or DCs treated with vitamin A derivatives are able to induce gut-homing properties in T-cells [123–126]. It was also recently established that migratory APCs imprint the integrin-mediated ability of T-cells to home to the CNS [129]. It may therefore be hypothesized that DCs and different DC-based vaccines can affect the homing abilities of tumor-specific T-cells, exhibiting differential abilities in terms of directing them to different tumors and other tissues.
In support of the notion that the migratory capacity of human, cancer-specific T-cells can be affected by DC-related factors (delivery of 'signal 4'), enhanced expression of functional cutaneous homing receptor (the ligand for the skin endothelial leucocyte adhesion molecule) and enhanced migration of effector CTLs to metastatic melanoma lesions in the skin could be induced by the treatment of patients with systemic IL-12 [130]. It has also been demonstrated that vaccination with monocyte-derived DCs can induce melanoma-specific T-cells that home to both the skin and visceral metastases [131].
The possibility that improved tumor homing may translate into better outcomes with active immunotherapies is supported by the observation that the level of T-cell infiltration is a strong independent prognostic marker of the survival of patients with melanoma [132] and colorectal cancer [133–135]. A dramatic survival advantage associated with CXCR3 (CXC chemokine receptor 3) expression by CTLs has also been observed in patients with advanced melanoma [136]. Therefore, the ability of DC vaccines to induce CXCR3-expressing CTLs is likely to contribute to their ability to act as effective vaccines against melanoma and potentially other tumors.
DC-produced chemokines: Avoiding regulatory T-cells and directing vaccination-induced effector cells to tumors
In addition to the ability of DC-based vaccines to induce desirable effector functions and the expression of a defined set of homing receptors on tumor-specific T-cells, another aspect that needs a thorough evaluation is the possibility to manipulate tumor-infiltrating DC vaccines to selectively express chemokines that attract (and thus preferentially activate) appropriate types of immune cells, such as Th1, NK cells and CTLs, while avoiding interaction with suppressor/regulatory cells. It was recently demonstrated that the conditions under which DCs mature imprint the ability to secrete different classes of chemokines as mature cells and thus, to selectively attract and interact with functionally distinct T-cell subsets [34]. DCs matured in the presence of PGE2 preferentially secrete CCL22/MDC (macrophage-derived chemokine) and attract Tregs [34], which may explain the previously reported preferential expansion of undesirable Tregs in patients vaccinated with PGE2-matured sDCs [27]. In contrast, the inclusion of IFNs in the DC maturation cocktails, particularly when combined with the absence of PGE2, suppresses CCL22 production and promotes the secretion of effector T-cell-attracting chemokines, such as CCL5 and CXCL10 (chemokine [C-X-C motif] ligand 10), as well as other CXCR3 ligands [34]. This ability to produce specific chemokines seems to be imprinted during maturation, as the chemokine expression remains stable even after removal of the original maturation stimulus [34]. Therefore, it is possible that the use of DCs matured in different environments, such as PGE2-matured sDCs and αDC1s matured in the presence of IFNs and other NK cell-replacing factors, by mimicking the conditions of acute infection, will preferentially amplify functionally different types of immunity.
As several tumor microenvironments are rich in PGE2 [30–33] and CCL22 [25], and/or are able to effectively recruit Tregs [25] rather than effector T-cells, it remains to be tested whether functional modulation of intratumoral DCs may reduce CCL22 levels and Treg infiltration. The possibility that tumor-specific chemokine modulation may enhance the overall effectiveness of cancer immunotherapy is indirectly supported by studies demonstrating that DCs in regressing tumors exhibit particularly high levels of CXCL9 expression and effectively attract CXCR3+ T-cells [137,138], and will be directly tested in upcoming clinical trials in colorectal cancer and melanoma.
Conclusion
Data from clinical trials of cancer vaccines, including first-generation DC-based vaccines, have suggested that such therapies may delay tumor progression and prolong the survival of patients with advanced cancer [16,17,103,104]. However, their activity in inducing tumor regression is still limited.
In addition to the more traditional role of DCs as carriers of `signal 1' and `signal 2', two additional aspects of DC biology are critical for the effectiveness of DC vaccines as a therapy for cancer; the induction of T-cells with the desirable effector functions and the ability to enter tumor tissues (that is the efficient delivery of `signal 3' and `signal 4') (Figure 2), and the preferential enhancement of the effector arm of immune responses without Treg hyperactivation. The latest advances in the area of DC biology should help in the development of vaccines able to induce T-cells with such properties, thus helping to bridge the gap between the effectiveness of therapeutic and protective vaccines.
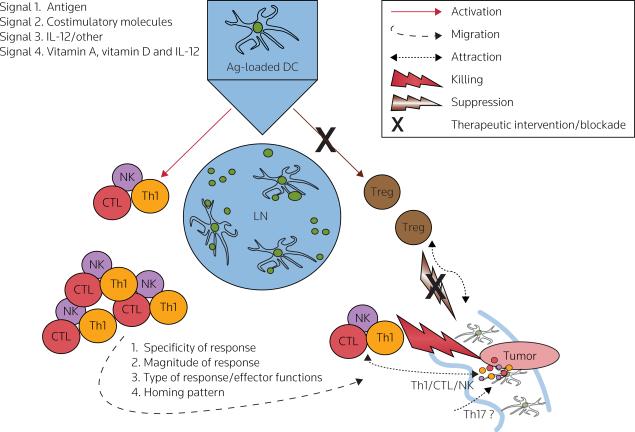
Figure 2. Roles of signals 1, 2, 3 and 4 in the induction of effective anticancer responses by DCs.
Dendritic cells (DCs) provide T-cells with an antigenic 'signal 1' and a costimulatory 'signal 2' [55–57], both of which are required for the activation and expansion of pathogen-specific T-cells. DCs also provide an additional, polarizing 'signal 3', driving the development of immune responses towards type-1 or type-2 immunity [51], and leading to differential activation of particular effector mechanisms, as well as different capabilities in inducing cancer rejection [51,55–64]. There are data indicating that DCs may also provide T-cells with an additional signal (tentatively termed 'signal 4'), which regulates organ-specific trafficking of immune cells [123–126,129,139,140]. The key role of DCs in regulating the expansion and acquisition of effector functions and/or tumor-relevant homing properties has suggested the possibility of exploiting these properties in the development of effective cancer immunotherapeutics, able to preferentially activate and expand the appropriate immune effector cells (T-helper-1 cells [Th1], CTLs, NK cells, and potentially Th17 cells), while avoiding activation of regulatory T-cells (Tregs), the undesirable phenomenon observed with current cancer vaccines [27]. Moreover, the effectiveness of therapeutic vaccines is likely to benefit from the development of strategies aimed at selective recruitment of effector T-cells into tumors, such as tumor-specific modulation of chemokine production. Red: effector cell-mediated immunity, brown: regulatory/suppressor cell-mediated immunity.
Ag antigen, LN lymph node
Acknowledgements
The author would like to thank Eve Blasko and Tina Kilgore for administrative assistance, and Drs Ravi Muthuswamy, Payal Watchmaker, Julie Urban and Drs Larissa Geskin, Hideho Okada, John Kirkwood, David Bartlett and Gurkamal Chatta for sharing the information on the status of their clinical trials. This work was supported by the NIH grants CA095128, CA114931, CA101944, CA121973 and CA132714.
References
•• of outstanding interest
• of special interest
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Thun MJ, Ries LA, Howe HL, Weir HK, Center MM, Ward E, Wu XC, Eheman C, Anderson R, Ajani UA, et al. Annual report to the nation on the status of cancer, 1975–2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst. 2008;100(23):1672–1694. doi: 10.1093/jnci/djn389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nauts HC. Bacteria and cancer: Antagonisms and benefits. Cancer Surv. 1989;8(4):713–723. [PubMed] [Google Scholar]
- 4.Lurquin C, Lethé B, De Plaen E, Corbière V, Théate I, van Baren N, Coulie PG, Boon T. Contrasting frequencies of antitumor and antivaccine T-cells in metastases of a melanoma patient vaccinated with a MAGE tumor antigen. J Exp Med. 2005;201(2):249–257. doi: 10.1084/jem.20041378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Godelaine D, Carrasco J, Lucas S, Karanikas V, Schuler-Thurner B, Coulie PG, Schuler G, Boon T, Van Pel A. Polyclonal CTL responses observed in melanoma patients vaccinated with dendritic cells pulsed with a MAGE-3.A1 peptide. J Immunol. 2003;171(9):4893–4897. doi: 10.4049/jimmunol.171.9.4893. [DOI] [PubMed] [Google Scholar]
- 6.Schlom J, Arlen PM, Gulley JL. Cancer vaccines: Moving beyond current paradigms. Clin Cancer Res. 2007;13(13):3776–3782. doi: 10.1158/1078-0432.CCR-07-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Discusses endpoints relevant to the evaluation of active cancer immunotherapies.
- 7.Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005;5(4):296–306. doi: 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- 8.Blattman JN, Greenberg PD. Cancer immunotherapy: A treatment for the masses. Science. 2004;305(5681):200–205. doi: 10.1126/science.1100369. [DOI] [PubMed] [Google Scholar]
- 9.Figdor CG, de Vries IJ, Lesterhuis WJ, Melief CJ. Dendritic cell immunotherapy: Mapping the way. Nat Med. 2004;10(5):475–480. doi: 10.1038/nm1039. [DOI] [PubMed] [Google Scholar]
- 10.Gilboa E. The promise of cancer vaccines. Nat Rev Cancer. 2004;4(5):401–411. doi: 10.1038/nrc1359. [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: Moving beyond current vaccines. Nat Med. 2004;10(9):909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Srivastava PK. Therapeutic cancer vaccines. Curr Opin Immunol. 2006;18(2):201–205. doi: 10.1016/j.coi.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Schlom J, Gulley JL, Arlen PM. Paradigm shifts in cancer vaccine therapy. Exp Biol Med (Maywood) 2008;233(5):522–534. doi: 10.3181/0708-MR-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel PH, Kockler DR. Sipuleucel-T: A vaccine for metastatic, asymptomatic, androgen-independent prostate cancer. Ann Pharmacother. 2008;42(1):91–98. doi: 10.1345/aph.1K429. [DOI] [PubMed] [Google Scholar]
- 15.Pilla L, Patuzzo R, Rivoltini L, Maio M, Pennacchioli E, Lamaj E, Maurichi A, Massarut S, Marchianò A, Santantonio C, Tosi D, et al. A phase II trial of vaccination with autologous, tumor-derived heat-shock protein peptide complexes Gp96, in combination with GM-CSF and interferon-α in metastatic melanoma patients. Cancer Immunol Immunother. 2006;55(8):958–968. doi: 10.1007/s00262-005-0084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Small EJ, Schellhammer PF, Higano CS, Redfern CH, Nemunaitis JJ, Valone FH, Verjee SS, Jones LA, Hershberg RM. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC-8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;24(19):3089–3094. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 17.Peoples GE, Holmes JP, Hueman MT, Mittendorf EA, Amin A, Khoo S, Dehqanzada ZA, Gurney JM, Woll MM, Ryan GB, Storrer CE, et al. Combined clinical trial results of a HER2/neu (E-75) vaccine for the prevention of recurrence in high-risk breast cancer patients: US Military Cancer Institute Clinical Trials Group Study I-01 and I-02. Clin Cancer Res. 2008;14(3):797–803. doi: 10.1158/1078-0432.CCR-07-1448. [DOI] [PubMed] [Google Scholar]
- 18.Mantovani A, Romero P, Palucka AK, Marincola FM. Tumor immunity: Effector response to tumor and role of the microenvironment. Lancet. 2008;371(9614):771–783. doi: 10.1016/S0140-6736(08)60241-X. [DOI] [PubMed] [Google Scholar]; • Reviews the interplay between the tumor microenvironment and the immune system in regulating progression and rejection of tumors.
- 19.Haring JS, Badovinac VP, Harty JT. Inflaming the CD8+ T-cell response. Immunity. 2006;25(1):19–29. doi: 10.1016/j.immuni.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Hartl D, Krauss-Etschmann S, Koller B, Hordijk PL, Kuijpers TW, Hoffmann F, Hector A, Eber E, Marcos V, Bittmann I, Eickelberg O, et al. Infiltrated neutrophils acquire novel chemokine receptor expression and chemokine responsiveness in chronic inflammatory lung diseases. J Immunol. 2008;181(11):8053–8067. doi: 10.4049/jimmunol.181.11.8053. [DOI] [PubMed] [Google Scholar]
- 21.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gabrilovich D. Mechanisms and functional significance of tumor-induced dendritic-cell defects. Nat Rev Immunol. 2004;4(12):941–952. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]; • Systematic review discussing the functional deficit of endogenous DCs in patients with cancer.
- 23.Gabrilovich D, Pisarev V. Tumor escape from immune response: Mechanisms and targets of activity. Curr Drug Targets. 2003;4(7):525–536. doi: 10.2174/1389450033490849. [DOI] [PubMed] [Google Scholar]
- 24.Zou W. Regulatory T-cells, tumor immunity and immunotherapy. Nat Rev Immunol. 2006;6(4):295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 25.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, et al. Specific recruitment of regulatory T-cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10(9):942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 26.Vieweg J, Su Z, Dahm P, Kusmartsev S. Reversal of tumor-mediated immunosuppression. Clin Cancer Res. 2007;13(2 Pt 2):727s–732s. doi: 10.1158/1078-0432.CCR-06-1924. [DOI] [PubMed] [Google Scholar]
- 27.Banerjee DK, Dhodapkar MV, Matayeva E, Steinman RM, Dhodapkar KM. Expansion of FOXP3high regulatory T-cells by human dendritic cells (DCs) in vitro and after injection of cytokine-matured DCs in myeloma patients. Blood. 2006;108(8):2655–2661. doi: 10.1182/blood-2006-03-011353. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Demonstrates that commonly used, DC-based vaccines generated in the presence of PGE2 may preferentially promote the expansion of Tregs.
- 28.Yang L, Carbone DP. Tumor-host immune interactions and dendritic cell dysfunction. Adv Cancer Res. 2004;92:13–27. doi: 10.1016/S0065-230X(04)92002-7. [DOI] [PubMed] [Google Scholar]
- 29.Pinzon-Charry A, Maxwell T, López JA. Dendritic cell dysfunction in cancer: A mechanism for immunosuppression. Immunol Cell Biol. 2005;83(5):451–461. doi: 10.1111/j.1440-1711.2005.01371.x. [DOI] [PubMed] [Google Scholar]
- 30.Uchida K, Schneider S, Yochim JM, Kuramochi H, Hayashi K, Takasaki K, Yang D, Danenberg KD, Danenberg PV. Intratumoral COX-2 gene expression is a predictive factor for colorectal cancer response to fluoropyrimidine-based chemotherapy. Clin Cancer Res. 2005;11(9):3363–3368. doi: 10.1158/1078-0432.CCR-04-1650. [DOI] [PubMed] [Google Scholar]
- 31.Williams C, Shattuck-Brandt RL, DuBois RN. The role of COX-2 in intestinal cancer. Ann NY Acad Sci. 1999;889:72–83. doi: 10.1111/j.1749-6632.1999.tb08725.x. [DOI] [PubMed] [Google Scholar]
- 32.Inaba T, Sano H, Kawahito Y, Hla T, Akita K, Toda M, Yamashina I, Inoue M, Nakada H. Induction of cyclooxygenase-2 in monocyte/macrophage by mucins secreted from colon cancer cells. Proc Natl Acad Sci USA. 2003;100(5):2736–2741. doi: 10.1073/pnas.0435410100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soumaoro LT, Uetake H, Higuchi T, Takagi Y, Enomoto M, Sugihara K. Cyclooxygenase-2 expression: A significant prognostic indicator for patients with colorectal cancer. Clin Cancer Res. 2004;10(24):8465–8471. doi: 10.1158/1078-0432.CCR-04-0653. [DOI] [PubMed] [Google Scholar]
- 34.Muthuswamy R, Urban J, Lee JJ, Reinhart TA, Bartlett D, Kalinski P. Ability of mature dendritic cells to interact with regulatory T-cells is imprinted during maturation. Cancer Res. 2008;68(14):5972–5978. doi: 10.1158/0008-5472.CAN-07-6818. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Demonstrates that the ability of DCs to preferentially interact with appropriate versus inappropriate types of immune cells (ie, effector T-cells versus Tregs) is imprinted during DC maturation.
- 35.Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI. Increased production of immature myeloid cells in cancer patients: A mechanism of immunosuppression in cancer. J Immunol. 2001;166(1):678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 36.Almand B, Resser JR, Lindman B, Nadaf S, Clark JI, Kwon ED, Carbone DP, Gabrilovich DI. Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res. 2000;6(5):1755–1766. [PubMed] [Google Scholar]
- 37.Gabrilovich DI, Velders MP, Sotomayor EM, Kast WM. Mechanism of immune dysfunction in cancer mediated by immature Gr-1+ myeloid cells. J Immunol. 2001;166(9):5398–5406. doi: 10.4049/jimmunol.166.9.5398. [DOI] [PubMed] [Google Scholar]
- 38.Young MR, Wright MA, Lozano Y, Prechel MM, Benefield J, Leonetti JP, Collins SL, Petruzzelli GJ. Increased recurrence and metastasis in patients whose primary head and neck squamous cell carcinomas secreted granulocyte-macrophage colony-stimulating factor and contained CD34+ natural suppressor cells. Int J Cancer. 1997;74(1):69–74. doi: 10.1002/(sici)1097-0215(19970220)74:1<69::aid-ijc12>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 39.Bell D, Chomarat P, Broyles D, Netto G, Harb GM, Lebecque S, Valladeau J, Davoust J, Palucka KA, Banchereau J. In breast carcinoma tissue, immature dendritic cells reside within the tumor, whereas mature dendritic cells are located in peritumoral areas. J Exp Med. 1999;190(10):1417–1426. doi: 10.1084/jem.190.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toriyama K, Wen DR, Paul E, Cochran AJ. Variations in the distribution, frequency, and phenotype of Langerhans cells during the evolution of malignant melanoma of the skin. J Invest Dermatol. 1993;100(3):269S–273S. doi: 10.1111/1523-1747.ep12470135. [DOI] [PubMed] [Google Scholar]
- 41.Troy AJ, Summers KL, Davidson PJ, Atkinson CH, Hart DN. Minimal recruitment and activation of dendritic cells within renal cell carcinoma. Clin Cancer Res. 1998;4(3):585–593. [PubMed] [Google Scholar]
- 42.Zou W, Machelon V, Coulomb-L'Hermin A, Borvak J, Nome F, Isaeva T, Wei S, Krzysiek R, Durand-Gasselin I, Gordon A, Pustilnik T, et al. Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nat Med. 2001;7(12):1339–1346. doi: 10.1038/nm1201-1339. [DOI] [PubMed] [Google Scholar]
- 43.Della Bella S, Gennaro M, Vaccari M, Ferraris C, Nicola S, Riva A, Clerici M, Greco M, Villa ML. Altered maturation of peripheral blood dendritic cells in patients with breast cancer. Br J Cancer. 2003;89(8):1463–1472. doi: 10.1038/sj.bjc.6601243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D, Yancey D, Zhang A, Dahm P, Chao N, Gilboa E, Vieweg J. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T-cells. J Clin Invest. 2005;115(12):3623–3633. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449(7161):419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 46.Steinman RM, Adams JC, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. IV. Identification and distribution in mouse spleen. J Exp Med. 1975;141(4):804–820. [PMC free article] [PubMed] [Google Scholar]
- 47.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973;137(5):1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. II. Functional properties in vitro. J Exp Med. 1974;139(2):380–397. doi: 10.1084/jem.139.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steinman RM, Lustig DS, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. 3. Functional properties in vivo. J Exp Med. 1974;139(6):1431–1445. doi: 10.1084/jem.139.6.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fujii S, Shimizu K, Hemmi H, Fukui M, Bonito AJ, Chen G, Franck RW, Tsuji M, Steinman RM. Glycolipid α-C-galactosylceramide is a distinct inducer of dendritic cell function during innate and adaptive immune responses of mice. Proc Natl Acad Sci USA. 2006;103(30):11252–11257. doi: 10.1073/pnas.0604812103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaliński P, Hilkens CM, Wierenga EA, Kapsenberg ML. T-cell priming by type-1 and type-2 polarized dendritic cells: The concept of a third signal. Immunol Today. 1999;20(12):561–567. doi: 10.1016/s0167-5699(99)01547-9. [DOI] [PubMed] [Google Scholar]
- 52.Kaliński P, Schuitemaker JH, Hilkens CM, Wierenga EA, Kapsenberg ML. Final maturation of dendritic cells is associated with impaired responsiveness to IFNγ and to bacterial IL-12 inducers: Decreased ability of mature dendritic cells to produce IL-12 during the interaction with Th cells. J Immunol. 1999;162(6):3231–3236. [PubMed] [Google Scholar]; • Demonstrated for the first time that DCs matured in the presence of inflammatory mediators or LPS can produce IL-12 only for a limited period of time and that they lose this capacity at later stages of the maturation process.
- 53.Vieira PL, de Jong EC, Wierenga EA, Kapsenberg ML, Kalinski P. Development of Th1-inducing capacity in myeloid dendritic cells requires environmental instruction. J Immunol. 2000;164(9):4507–4512. doi: 10.4049/jimmunol.164.9.4507. [DOI] [PubMed] [Google Scholar]; • Demonstrated for the first time that DCs matured in the presence of IFNγ avoid `exhaustion', retain their ability to produce high levels of IL-12 upon interaction with T-cells and exhibit a strongly enhanced ability to induce type-1 immunity.
- 54.Kaliński P, Schuitemaker JH, Hilkens CM, Kapsenberg ML. Prostaglandin E2 induces the final maturation of IL-12-deficient CD1a+CD83+ dendritic cells: The levels of IL-12 are determined during the final dendritic cell maturation and are resistant to further modulation. J Immunol. 1998;161(6):2804–2809. [PubMed] [Google Scholar]
- 55.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 56.Schuler G, Schuler-Thurner B, Steinman RM. The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol. 2003;15(2):138–147. doi: 10.1016/s0952-7915(03)00015-3. [DOI] [PubMed] [Google Scholar]
- 57.Schuler G, Steinman RM. Dendritic cells as adjuvants for immune-mediated resistance to tumors. J Exp Med. 1997;186(8):1183–1187. doi: 10.1084/jem.186.8.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003;3(12):984–993. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- 59.Ikeda H, Chamoto K, Tsuji T, Suzuki Y, Wakita D, Takeshima T, Nishimura T. The critical role of type-1 innate and acquired immunity in tumor immunotherapy. Cancer Sci. 2004;95(9):697–703. doi: 10.1111/j.1349-7006.2004.tb03248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pulendran B. Modulating Th1/Th2 responses with microbes, dendritic cells, and pathogen recognition receptors. Immunol Res. 2004;29(1–3):187–196. doi: 10.1385/IR:29:1-3:187. [DOI] [PubMed] [Google Scholar]
- 61.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3(2):133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 62.Palucka K, Banchereau J. How dendritic cells and microbes interact to elicit or subvert protective immune responses. Curr Opin Immunol. 2002;14(4):420–431. doi: 10.1016/s0952-7915(02)00365-5. [DOI] [PubMed] [Google Scholar]
- 63.Czerniecki BJ, Cohen PA, Faries M, Xu S, Roros JG, Bedrosian I. Diverse functional activity of CD83+ monocyte-derived dendritic cells and the implications for cancer vaccines. Crit Rev Immunol. 2001;21(1–3):157–178. [PubMed] [Google Scholar]
- 64.Kalinski P, Moser M. Consensual immunity: Success-driven development of T-helper-1 and T-helper-2 responses. Nat Rev Immunol. 2005;5(3):251–260. doi: 10.1038/nri1569. [DOI] [PubMed] [Google Scholar]
- 65.Fernandez NC, Lozier A, Flament C, Ricciardi-Castagnoli P, Bellet D, Suter M, Perricaudet M, Tursz T, Maraskovsky E, Zitvogel L. Dendritic cells directly trigger NK cell functions: Cross-talk relevant in innate antitumor immune responses in vivo. Nat Med. 1999;5(4):405–411. doi: 10.1038/7403. [DOI] [PubMed] [Google Scholar]
- 66.Gustafsson K, Ingelsten M, Bergqvist L, Nyström J, Andersson B, Karlsson-Parra A. Recruitment and activation of natural killer cells in vitro by a human dendritic cell vaccine. Cancer Res. 2008;68(14):5965–5971. doi: 10.1158/0008-5472.CAN-07-6494. [DOI] [PubMed] [Google Scholar]
- 67.Dhodapkar MV, Steinman RM, Sapp M, Desai H, Fossella C, Krasovsky J, Donahoe SM, Dunbar PR, Cerundolo V, Nixon DF, Bhardwaj N. Rapid generation of broad T-cell immunity in humans after a single injection of mature dendritic cells. J Clin Invest. 1999;104(2):173–180. doi: 10.1172/JCI6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.de Vries IJ, Lesterhuis WJ, Scharenborg NM, Engelen LP, Ruiter DJ, Gerritsen MJ, Croockewit S, Britten CM, Torensma R, Adema GJ, Figdor CG, et al. Maturation of dendritic cells is a prerequisite for inducing immune responses in advanced melanoma patients. Clin Cancer Res. 2003;9(14):5091–5100. [PubMed] [Google Scholar]
- 69.Adema GJ, de Vries IJ, Punt CJ, Figdor CG. Migration of dendritic cell-based cancer vaccines: In vivo veritas? Curr Opin Immunol. 2005;17(2):170–174. doi: 10.1016/j.coi.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 70.De Vries IJ, Krooshoop DJ, Scharenborg NM, Lesterhuis WJ, Diepstra JH, Van Muijen GN, Strijk SP, Ruers TJ, Boerman OC, Oyen WJ, Adema GJ, et al. Effective migration of antigen-pulsed dendritic cells to lymph nodes in melanoma patients is determined by their maturation state. Cancer Res. 2003;63(1):12–17. [PubMed] [Google Scholar]
- 71.Zitvogel L, Mayordomo JI, Tjandrawan T, DeLeo AB, Clarke MR, Lotze MT, Storkus WJ. Therapy of murine tumors with tumor peptide-pulsed dendritic cells: Dependence on T-cells, B7 costimulation, and T helper cell 1-associated cytokines. J Exp Med. 1996;183(1):87–97. doi: 10.1084/jem.183.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zitvogel L, Robbins PD, Storkus WJ, Clarke MR, Maeurer MJ, Campbell RL, Davis CG, Tahara H, Schreiber RD, Lotze MT. Interleukin-12 and B7.1 costimulation co-operate in the induction of effective antitumor immunity and therapy of established tumors. Eur J Immunol. 1996;26(6):1335–1341. doi: 10.1002/eji.1830260624. [DOI] [PubMed] [Google Scholar]
- 73.Furumoto K, Arii S, Yamasaki S, Mizumoto M, Mori A, Inoue N, Isobe N, Imamura M. Spleen-derived dendritic cells engineered to enhance interleukin-12 production elicit therapeutic antitumor immune responses. Int J Cancer. 2000;87(5):665–672. [PubMed] [Google Scholar]
- 74.Furumoto K, Mori A, Yamasaki S, Inoue N, Yang W, Nakau M, Yasuda S, Arii S, Imamura M. Interleukin-12-gene transduction makes DCs from tumor-bearing mice an effective inducer of tumor-specific immunity in a peritoneal dissemination model. Immunol Lett. 2002;83(1):13–20. doi: 10.1016/s0165-2478(02)00071-8. [DOI] [PubMed] [Google Scholar]
- 75.Nishioka Y, Hirao M, Robbins PD, Lotze MT, Tahara H. Induction of systemic and therapeutic antitumor immunity using intratumoral injection of dendritic cells genetically modified to express interleukin-12. Cancer Res. 1999;59(16):4035–4041. [PubMed] [Google Scholar]
- 76.Okada N, Iiyama S, Okada Y, Mizuguchi H, Hayakawa T, Nakagawa S, Mayumi T, Fujita T, Yamamoto A. Immunological properties and vaccine efficacy of murine dendritic cells simultaneously expressing melanoma-associated antigen and interleukin-12. Cancer Gene Ther. 2005;12(1):72–83. doi: 10.1038/sj.cgt.7700772. [DOI] [PubMed] [Google Scholar]
- 77.Redlinger RE, Jr, Mailliard RB, Barksdale EM., Jr Advanced neuroblastoma impairs dendritic cell function in adoptive immunotherapy. J Pediatr Surg. 2003;38(6):857–862. doi: 10.1016/s0022-3468(03)00111-8. [DOI] [PubMed] [Google Scholar]
- 78.Satoh Y, Esche C, Gambotto A, Shurin GV, Yurkovetsky ZR, Robbins PD, Watkins SC, Todo S, Herberman RB, Lotze MT, Shurin MR. Local administration of IL-12-transfected dendritic cells induces antitumor immune responses to colon adenocarcinoma in the liver in mice. J Exp Ther Oncol. 2002;2(6):337–349. doi: 10.1046/j.1359-4117.2002.01050.x. [DOI] [PubMed] [Google Scholar]
- 79.Shimizu T, Berhanu A, Redlinger RE, Jr, Watkins S, Lotze MT, Barksdale EM., Jr Interleukin-12 transduced dendritic cells induce regression of established murine neuroblastoma. J Pediatr Surg. 2001;36(8):1285–1292. doi: 10.1053/jpsu.2001.25796. [DOI] [PubMed] [Google Scholar]
- 80.Yamanaka R, Zullo SA, Ramsey J, Yajima N, Tsuchiya N, Tanaka R, Blaese M, Xanthopoulos KG. Marked enhancement of antitumor immune responses in mouse brain tumor models by genetically modified dendritic cells producing Semliki Forest virus-mediated interleukin-12. J Neurosurg. 2002;97(3):611–618. doi: 10.3171/jns.2002.97.3.0611. [DOI] [PubMed] [Google Scholar]
- 81.Zhang S, Zeng G, Wilkes DS, Reed GE, McGarry RC, Eble JN, Cheng L. Dendritic cells transfected with interleukin-12 and pulsed with tumor extract inhibit growth of murine prostatic carcinoma in vivo. Prostate. 2003;55(4):292–298. doi: 10.1002/pros.10246. [DOI] [PubMed] [Google Scholar]
- 82.Hsu FJ, Benike C, Fagnoni F, Liles TM, Czerwinski D, Taidi B, Engleman EG, Levy R. Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cells. Nat Med. 1996;2(1):52–58. doi: 10.1038/nm0196-52. [DOI] [PubMed] [Google Scholar]; • Demonstrated for the first time the immunological and clinical effectiveness of DC-based therapeutic cancer vaccines in patients with hematological malignancies.
- 83.Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D. Vaccination of melanoma patients with peptide-or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4(3):328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]; • Demonstrated for the first time the immunological and clinical effectiveness of DC-based therapeutic cancer vaccines against solid tumors.
- 84.Jonuleit H, Kühn U, Müller G, Steinbrink K, Paragnik L, Schmitt E, Knop J, Enk AH. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol. 1997;27(12):3135–3142. doi: 10.1002/eji.1830271209. [DOI] [PubMed] [Google Scholar]
- 85.Langenkamp A, Messi M, Lanzavecchia A, Sallusto F. Kinetics of dendritic cell activation: Impact on priming of Th1, Th2 and non-polarized T-cells. Nat Immunol. 2000;1(4):311–316. doi: 10.1038/79758. [DOI] [PubMed] [Google Scholar]
- 86.Mailliard RB, Wankowicz-Kalinska A, Cai Q, Wesa A, Hilkens CM, Kapsenberg ML, Kirkwood JM, Storkus WJ, Kalinski P. α-Type-1 polarized dendritic cells: A novel immunization tool with optimized CTL-inducing activity. Cancer Res. 2004;64(17):5934–5937. doi: 10.1158/0008-5472.CAN-04-1261. [DOI] [PubMed] [Google Scholar]
- 87.Luft T, Jefford M, Luetjens P, Toy T, Hochrein H, Masterman KA, Maliszewski C, Shortman K, Cebon J, Maraskovsky E. Functionally distinct dendritic cell (DC) populations induced by physiologic stimuli: Prostaglandin E2 regulates the migratory capacity of specific DC subsets. Blood. 2002;100(4):1362–1372. doi: 10.1182/blood-2001-12-0360. [DOI] [PubMed] [Google Scholar]
- 88.Scandella E, Men Y, Gillessen S, Förster R, Groettrup M. Prostaglandin E2 is a key factor for CCR7 surface expression and migration of monocyte-derived dendritic cells. Blood. 2002;100(4):1354–1361. doi: 10.1182/blood-2001-11-0017. [DOI] [PubMed] [Google Scholar]
- 89.Kalinski P, Giermasz A, Nakamura Y, Basse P, Storkus WJ, Kirkwood JM, Mailliard RB. Helper role of NK cells during the induction of anticancer responses by dendritic cells. Mol Immunol. 2005;42(4):535–539. doi: 10.1016/j.molimm.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 90.Kalinski P, Mailliard RB, Giermasz A, Zeh HJ, Basse P, Bartlett DL, Kirkwood JM, Lotze MT, Herberman RB. Natural killer-dendritic cell cross-talk in cancer immunotherapy. Expert Opin Biol Ther. 2005;5(10):1303–1315. doi: 10.1517/14712598.5.10.1303. [DOI] [PubMed] [Google Scholar]
- 91.Mailliard RB, Egawa S, Cai Q, Kalinska A, Bykovskaya SN, Lotze MT, Kapsenberg ML, Storkus WJ, Kalinski P. Complementary dendritic cell-activating function of CD8+ and CD4+ T-cells: Helper role of CD8+ T-cells in the development of T-helper type 1 responses. J Exp Med. 2002;195(4):473–483. doi: 10.1084/jem.20011662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mailliard RB, Son YI, Redlinger R, Coates PT, Giermasz A, Morel PA, Storkus WJ, Kalinski P. Dendritic cells mediate NK cell help for Th1 and CTL responses: Two-signal requirement for the induction of NK cell helper function. J Immunol. 2003;171(5):2366–2373. doi: 10.4049/jimmunol.171.5.2366. [DOI] [PubMed] [Google Scholar]
- 93.Xu S, Koski GK, Faries M, Bedrosian I, Mick R, Maeurer M, Cheever MA, Cohen PA, Czerniecki BJ. Rapid high efficiency sensitization of CD8+ T-cells to tumor antigens by dendritic cells leads to enhanced functional avidity and direct tumor recognition through an IL-12-dependent mechanism. J Immunol. 2003;171(5):2251–2261. doi: 10.4049/jimmunol.171.5.2251. [DOI] [PubMed] [Google Scholar]
- 94.Wesa A, Kalinski P, Kirkwood JM, Tatsumi T, Storkus WJ. Polarized type-1 dendritic cells (DC1) producing high levels of IL-12 family members rescue patient Th1-type antimelanoma CD4+ T-cell responses in vitro. J Immunother. 2007;30(1):75–82. doi: 10.1097/01.cji.0000211316.15278.6e. [DOI] [PubMed] [Google Scholar]
- 95.Kalinski P, Nakamura Y, Watchmaker P, Giermasz A, Muthuswamy R, Mailliard RB. Helper roles of NK and CD8+ T-cells in the induction of tumor immunity. Polarized dendritic cells as cancer vaccines. Immunol Res. 2006;36(1–3):137–146. doi: 10.1385/IR:36:1:137. [DOI] [PubMed] [Google Scholar]
- 96.Ten Brinke A, Karsten ML, Dieker MC, Zwaginga JJ, van Ham SM. The clinical grade maturation cocktail monophosphoryl lipid A plus IFNγ generates monocyte-derived dendritic cells with the capacity to migrate and induce Th1 polarization. Vaccine. 2007;25(41):7145–7152. doi: 10.1016/j.vaccine.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 97.Tarbell KV, Petit L, Zuo X, Toy P, Luo X, Mqadmi A, Yang H, Suthanthiran M, Mojsov S, Steinman RM. Dendritic cell-expanded, islet-specific CD4+ CD25+ CD62L+ regulatory T-cells restore normoglycemia in diabetic NOD mice. J Exp Med. 2007;204(1):191–201. doi: 10.1084/jem.20061631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yamazaki S, Inaba K, Tarbell KV, Steinman RM. Dendritic cells expand antigen-specific Foxp3+ CD25+ CD4+ regulatory T-cells including suppressors of alloreactivity. Immunol Rev. 2006;212:314–329. doi: 10.1111/j.0105-2896.2006.00422.x. [DOI] [PubMed] [Google Scholar]
- 99.Yamazaki S, Iyoda T, Tarbell K, Olson K, Velinzon K, Inaba K, Steinman RM. Direct expansion of functional CD25+ CD4+ regulatory T-cells by antigen-processing dendritic cells. J Exp Med. 2003;198(2):235–247. doi: 10.1084/jem.20030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dhodapkar MV, Steinman RM. Antigen-bearing immature dendritic cells induce peptide-specific CD8+ regulatory T-cells in vivo in humans. Blood. 2002;100(1):174–177. doi: 10.1182/blood.v100.1.174. [DOI] [PubMed] [Google Scholar]
- 101.Lee JJ, Foon KA, Mailliard RB, Muthuswamy R, Kalinski P. Type 1-polarized dendritic cells loaded with autologous tumor are a potent immunogen against chronic lymphocytic leukemia. J Leukoc Biol. 2008;84(1):319–825. doi: 10.1189/jlb.1107737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nestle FO, Farkas A, Conrad C. Dendritic-cell-based therapeutic vaccination against cancer. Curr Opin Immunol. 2005;17(2):163–169. doi: 10.1016/j.coi.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 103.Sipuleucel-T: APC-8015, APC-8015, prostate cancer vaccine – Dendreon. Drugs R D. 2006;7(3):197–201. doi: 10.2165/00126839-200607030-00006. No authors listed. [DOI] [PubMed] [Google Scholar]
- 104.Harzstark AL, Small EJ. Immunotherapy for prostate cancer using antigen-loaded antigen-presenting cells: APC-8015 (Provenge) Expert Opin Biol Ther. 2007;7(8):1275–1280. doi: 10.1517/14712598.7.8.1275. [DOI] [PubMed] [Google Scholar]
- 105.Schadendorf D, Ugurel S, Schuler-Thurner B, Nestle FO, Enk A, Bröcker EB, Grabbe S, Rittgen W, Edler L, Sucker A, Zimpfer-Rechner C, et al. Dacarbazine (DTIC) versus vaccination with autologous peptide-pulsed dendritic cells (DC) in first-line treatment of patients with metastatic melanoma: A randomized phase III trial of the DC study group of the DeCOG. Ann Oncol. 2006;17(4):563–570. doi: 10.1093/annonc/mdj138. [DOI] [PubMed] [Google Scholar]
- 106.Kaliński P, Vieira PL, Schuitemaker JH, de Jong EC, Kapsenberg ML. Prostaglandin E2 is a selective inducer of interleukin-12 p40 (IL-12p40) production and an inhibitor of bioactive IL-12p70 heterodimer. Blood. 2001;97(11):3466–3469. doi: 10.1182/blood.v97.11.3466. [DOI] [PubMed] [Google Scholar]
- 107.Dubsky P, Saito H, Leogier M, Dantin C, Connolly JE, Banchereau J, Palucka AK. IL-15-induced human DC efficiently prime melanoma-specific naive CD8+ T-cells to differentiate into CTL. Eur J Immunol. 2007;37(6):1678–1690. doi: 10.1002/eji.200636329. [DOI] [PubMed] [Google Scholar]
- 108.Nguyen LT, Radhakrishnan S, Ciric B, Tamada K, Shin T, Pardoll DM, Chen L, Rodriguez M, Pease LR. Cross-linking the B7 family molecule B7-DC directly activates immune functions of dendritic cells. J Exp Med. 2002;196(10):1393–1398. doi: 10.1084/jem.20021466. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 109.Wang S, Yang J, Qian J, Wezeman M, Kwak LW, Yi Q. Tumor evasion of the immune system: Inhibiting p38 MAPK signaling restores the function of dendritic cells in multiple myeloma. Blood. 2006;107(6):2432–2439. doi: 10.1182/blood-2005-06-2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jarnicki AG, Conroy H, Brereton C, Donnelly G, Toomey D, Walsh K, Sweeney C, Leavy O, Fletcher J, Lavelle EC, Dunne P, et al. Attenuating regulatory T-cell induction by TLR agonists through inhibition of p38 MAPK signaling in dendritic cells enhances their efficacy as vaccine adjuvants and cancer immunotherapeutics. J Immunol. 2008;180(6):3797–3806. doi: 10.4049/jimmunol.180.6.3797. [DOI] [PubMed] [Google Scholar]
- 111.Hilkens CM, Kalinski P, de Boer M, Kapsenberg ML. Human dendritic cells require exogenous interleukin-12-inducing factors to direct the development of naive T-helper cells toward the Th1 phenotype. Blood. 1997;90(5):1920–1926. [PubMed] [Google Scholar]
- 112.Snijders A, Kalinski P, Hilkens CM, Kapsenberg ML. High-level IL-12 production by human dendritic cells requires two signals. Int Immunol. 1998;10(11):1593–1598. doi: 10.1093/intimm/10.11.1593. [DOI] [PubMed] [Google Scholar]
- 113.Schulz O, Edwards AD, Schito M, Aliberti J, Manickasingham S, Sher A, Reis e Sousa C. CD40 triggering of heterodimeric IL-12p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity. 2000;13(4):453–462. doi: 10.1016/s1074-7613(00)00045-5. [DOI] [PubMed] [Google Scholar]
- 114.Spörri R, Reis e Sousa C. Inflammatory mediators are insufficient for full dendritic cell activation and promote expansion of CD4+ T-cell populations lacking helper function. Nat Immunol. 2005;6(2):163–170. doi: 10.1038/ni1162. [DOI] [PubMed] [Google Scholar]
- 115.Berg EL, Goldstein LA, Jutila MA, Nakache M, Picker LJ, Streeter PR, Wu NW, Zhou D, Butcher EC. Homing receptors and vascular addressins: Cell adhesion molecules that direct lymphocyte traffic. Immunol Rev. 1989;108:5–18. doi: 10.1111/j.1600-065x.1989.tb00010.x. [DOI] [PubMed] [Google Scholar]
- 116.Picker LJ, Kishimoto TK, Smith CW, Warnock RA, Butcher EC. ELAM-1 is an adhesion molecule for skin-homing T-cells. Nature. 1991;349(6312):796–799. doi: 10.1038/349796a0. [DOI] [PubMed] [Google Scholar]
- 117.Picker LJ, Terstappen LW, Rott LS, Streeter PR, Stein H, Butcher EC. Differential expression of homing-associated adhesion molecules by T-cell subsets in man. J Immunol. 1990;145(10):3247–3255. [PubMed] [Google Scholar]
- 118.Picker LJ, Treer JR, Ferguson-Darnell B, Collins PA, Bergstresser PR, Terstappen LW. Control of lymphocyte recirculation in man. II. Differential regulation of the cutaneous lymphocyte-associated antigen, a tissue-selective homing receptor for skin-homing T-cells. J Immunol. 1993;150(3):1122–1136. [PubMed] [Google Scholar]
- 119.Sallusto F, Lanzavecchia A. Understanding dendritic cell and T-lymphocyte traffic through the analysis of chemokine receptor expression. Immunol Rev. 2000;177:134–140. doi: 10.1034/j.1600-065x.2000.17717.x. [DOI] [PubMed] [Google Scholar]
- 120.Sallusto F, Mackay CR. Chemoattractants and their receptors in homeostasis and inflammation. Curr Opin Immunol. 2004;16(6):724–731. doi: 10.1016/j.coi.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 121.Sallusto F, Mackay CR, Lanzavecchia A. The role of chemokine receptors in primary, effector, and memory immune responses. Annu Rev Immunol. 2000;18:593–620. doi: 10.1146/annurev.immunol.18.1.593. [DOI] [PubMed] [Google Scholar]
- 122.Johansson-Lindbom B, Svensson M, Wurbel MA, Malissen B, Márquez G, Agace W. Selective generation of gut tropic T-cells in gut-associated lymphoid tissue (GALT): Requirement for GALT dendritic cells and adjuvant. J Exp Med. 2003;198(6):963–969. doi: 10.1084/jem.20031244. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Elegantly demonstrates that the ability of T-cells to enter gut tissues can be imprinted in T-cells by DCs.
- 123.Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, Von Andrian UH. Selective imprinting of gut-homing T-cells by Peyer's patch dendritic cells. Nature. 2003;424(6944):88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 124.Mora JR, Cheng G, Picarella D, Briskin M, Buchanan N, von Andrian UH. Reciprocal and dynamic control of CD8 T-cell homing by dendritic cells from skin- and gut-associated lymphoid tissues. J Exp Med. 2005;201(2):303–316. doi: 10.1084/jem.20041645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mora JR, von Andrian UH. Retinoic acid: An educational 'vitamin elixir' for gut-seeking T-cells. Immunity. 2004;21(4):458–460. doi: 10.1016/j.immuni.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 126.Stagg AJ, Kamm MA, Knight SC. Intestinal dendritic cells increase T-cell expression of α4β7 integrin. Eur J Immunol. 2002;32(5):1445–1454. doi: 10.1002/1521-4141(200205)32:5<1445::AID-IMMU1445>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 127.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T-cells. Immunity. 2004;21(4):527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 128.Sigmundsdottir H, Pan J, Debes GF, Alt C, Habtezion A, Soler D, Butcher EC. DCs metabolize sunlight-induced vitamin D3 to 'program' T-cell attraction to the epidermal chemokine CCL27. Nat Immunol. 2007;8(3):285–293. doi: 10.1038/ni1433. [DOI] [PubMed] [Google Scholar]; • Demonstrates that skin-homing properties can be imprinted in T-cells by DCs via a mechanism involving vitamin D.
- 129.Calzascia T, Masson F, Di Berardino-Besson W, Contassot E, Wilmotte R, Aurrand-Lions M, Rüegg C, Dietrich PY, Walker PR. Homing phenotypes of tumor-specific CD8 T cells are predetermined at the tumor site by crosspresenting APCs. Immunity. 2005;22(2):175–184. doi: 10.1016/j.immuni.2004.12.008. [DOI] [PubMed] [Google Scholar]; • Elegantly demonstrates that CNS-homing properties can be imprinted in T-cells by APCs.
- 130.Mortarini R, Borri A, Tragni G, Bersani I, Vegetti C, Bajetta E, Pilotti S, Cerundolo V, Anichini A. Peripheral burst of tumor-specific cytotoxic T-lymphocytes and infiltration of metastatic lesions by memory CD8+ T-cells in melanoma patients receiving interleukin 12. Cancer Res. 2000;60(13):3559–3568. [PubMed] [Google Scholar]
- 131.Berger TG, Haendle I, Schrama D, Lüftl M, Bauer N, Pedersen LØ, Schuler-Thurner B, Hohenberger W, Straten P, Pt, Schuler G, Becker JC. Circulation and homing of melanoma-reactive T-cells to both cutaneous and visceral metastases after vaccination with monocyte-derived dendritic cells. Int J Cancer. 2004;111(2):229–237. doi: 10.1002/ijc.20238. [DOI] [PubMed] [Google Scholar]
- 132.Piras F, Colombari R, Minerba L, Murtas D, Floris C, Maxia C, Corbu A, Perra MT, Sirigu P. The predictive value of CD8, CD4, CD68, and human leukocyte antigen-D-related cells in the prognosis of cutaneous malignant melanoma with vertical growth phase. Cancer. 2005;104(6):1246–1254. doi: 10.1002/cncr.21283. [DOI] [PubMed] [Google Scholar]
- 133.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoué F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]; •• Demonstrates that the level of tumor T-cell infiltration (T-cell density and effector phenotype) can act as a predictive marker for the long-term survival in patients with colorectal cancer.
- 134.Galon J, Fridman WH, Pagès F. The adaptive immunologic microenvironment in colorectal cancer: A novel perspective. Cancer Res. 2007;67(5):1883–1886. doi: 10.1158/0008-5472.CAN-06-4806. [DOI] [PubMed] [Google Scholar]
- 135.Pagès F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, Meatchi T, et al. Effector memory T-cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353(25):2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 136.Mullins IM, Slingluff CL, Lee JK, Garbee CF, Shu J, Anderson SG, Mayer ME, Knaus WA, Mullins DW. CXC chemokine receptor 3 expression by activated CD8+ T cells is associated with survival in melanoma patients with stage III disease. Cancer Res. 2004;64(21):7697–7701. doi: 10.1158/0008-5472.CAN-04-2059. [DOI] [PubMed] [Google Scholar]
- 137.Kunz M, Toksoy A, Goebeler M, Engelhardt E, Bröcker E, Gillitzer R. Strong expression of the lymphoattractant C-X-C chemokine Mig is associated with heavy infiltration of T-cells in human malignant melanoma. J Pathol. 1999;189(4):552–558. doi: 10.1002/(SICI)1096-9896(199912)189:4<552::AID-PATH469>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 138.Ohtani H, Jin Z, Takegawa S, Nakayama T, Yoshie O. Abundant expression of CXCL9 (MIG) by stromal cells that include dendritic cells and accumulation of CXCR3+ T-cells in lymphocyte-rich gastric carcinoma. J Pathol. 2009;217(1):21–31. doi: 10.1002/path.2448. [DOI] [PubMed] [Google Scholar]
- 139.Schaerli P, Loetscher P, Moser B. Cutting edge: Induction of follicular homing precedes effector Th cell development. J Immunol. 2001;167(11):6082–6086. doi: 10.4049/jimmunol.167.11.6082. [DOI] [PubMed] [Google Scholar]
- 140.Weninger W, Manjunath N, von Andrian UH. Migration and differentiation of CD8+ T-cells. Immunol Rev. 2002;186:221–233. doi: 10.1034/j.1600-065x.2002.18618.x. [DOI] [PubMed] [Google Scholar]