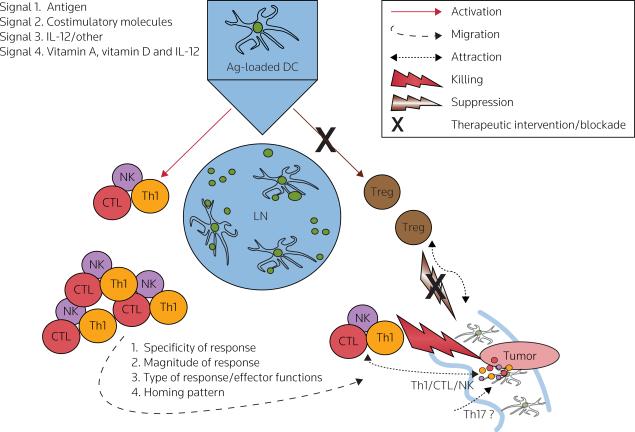
Figure 2. Roles of signals 1, 2, 3 and 4 in the induction of effective anticancer responses by DCs.
Dendritic cells (DCs) provide T-cells with an antigenic 'signal 1' and a costimulatory 'signal 2' [55–57], both of which are required for the activation and expansion of pathogen-specific T-cells. DCs also provide an additional, polarizing 'signal 3', driving the development of immune responses towards type-1 or type-2 immunity [51], and leading to differential activation of particular effector mechanisms, as well as different capabilities in inducing cancer rejection [51,55–64]. There are data indicating that DCs may also provide T-cells with an additional signal (tentatively termed 'signal 4'), which regulates organ-specific trafficking of immune cells [123–126,129,139,140]. The key role of DCs in regulating the expansion and acquisition of effector functions and/or tumor-relevant homing properties has suggested the possibility of exploiting these properties in the development of effective cancer immunotherapeutics, able to preferentially activate and expand the appropriate immune effector cells (T-helper-1 cells [Th1], CTLs, NK cells, and potentially Th17 cells), while avoiding activation of regulatory T-cells (Tregs), the undesirable phenomenon observed with current cancer vaccines [27]. Moreover, the effectiveness of therapeutic vaccines is likely to benefit from the development of strategies aimed at selective recruitment of effector T-cells into tumors, such as tumor-specific modulation of chemokine production. Red: effector cell-mediated immunity, brown: regulatory/suppressor cell-mediated immunity.
Ag antigen, LN lymph node